N-Phenylhydroxylamine
Synonym(s):N-Hydroxyaniline;N-Hydroxybenzenamine
- CAS NO.:100-65-2
- Empirical Formula: C6H7NO
- Molecular Weight: 109.13
- MDL number: MFCD00045718
- EINECS: 202-875-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
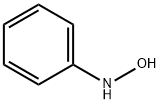
What is N-Phenylhydroxylamine?
Chemical properties
tan powder or crystals
The Uses of N-Phenylhydroxylamine
Manufacture of cupferron.
The Uses of N-Phenylhydroxylamine
N-Phenylhydroxylamine can be used as a starting material for the synthesis of:
- 2-alkylindoles by treating with aliphatic terminal alkynes using gold catalyst via sequential 3,3-rearrangements and cyclodehydrations.
- Isoxazolidines by reacting with aldehydes and α, β-unsaturated aldehydes via a three-component one-pot catalytic reaction.
- Tetrahydro-1,2-oxazines by treating with an aldehyde and cyclopropane via homo 3+2 dipolar cycloaddition reaction.
Definition
ChEBI: N-phenylhydroxylamine is an N-substituted amine that is a derivative of aniline in which one of the amino hydrogen atoms is replaced with a hydroxy substituent.
Preparation
To a dispersion of 180 gm (2.75 gm-atom) of zinc dust in 500 ml of 50% aqueous ethanol, with vigorous stirring, is added 130 ml (156 gm, 1.27 mole) of nitrobenzene. The reaction is initiated by the dropwise addition of an aqueous ammonium chloride solution and can thereafter be maintained at a controllable rate by the cautious addition of the remaining ammonium chloride solution. The reaction temperature rises during this reduction step. Once reflux has subsided, the basic zinc salts are filtered off using a sintered glass funnel. The light green filtrate is cooled in an ice-salt mixture to precipitate the product. The yield is 100 gm (72.2%), m.p. 81°C.
The reduction of 2-methyl-2-nitropropane is carried out in a similar manner at 10-20°C to afford a 68% yield of N-t-butylhydroxylamine (m.p. 60-62°C).
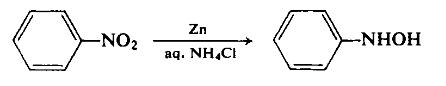
General Description
Tan to brown crystals.
Air & Water Reactions
Soluble in hot water.
Reactivity Profile
N-Phenylhydroxylamine neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides.
Fire Hazard
Flash point data for N-Phenylhydroxylamine are not available but N-Phenylhydroxylamine is probably nonflammable.
Safety Profile
Poison by ingestion and subcutaneous routes. Human systemic effects by skin contact: primary irritation. Preparative hazard. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx.
Purification Methods
Impure base deteriorates rapidly. Crystallise it from H2O, *C6H6 or *C6H6/pet ether (40-60o). The picrate has m 186o (from EtOH), and the benzenesulfonate salt has m 70o (dec )(EtOH/*C6H6). [Beilstein 15 H 2, 15 I 3, 15, II 4, 15 III 5. 15 IV 4.]
Properties of N-Phenylhydroxylamine
| Melting point: | 80-84℃ |
| Boiling point: | 204.59°C (rough estimate) |
| Density | 1.1143 (rough estimate) |
| refractive index | 1.5444 (estimate) |
| storage temp. | -20°C |
| pka | 9.00±0.70(Predicted) |
| form | solid |
| color | Light brown |
| Water Solubility | 20g/L(5 ºC) |
| Stability: | Unstable - deteriorates with storage. Incompatible with strong oxidizing agents. |
| EPA Substance Registry System | Phenylhydroxylamine (100-65-2) |
Safety information for N-Phenylhydroxylamine
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
| Precautionary Statement Codes |
P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for N-Phenylhydroxylamine
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![Ethyl 3-[N-(phenyl)amino]-3-oxopropionate](https://img.chemicalbook.in/CAS/20150408/GIF/53341-66-5.gif)
You may like
-
 N-Phenylhydroxylamine 90% CAS 100-65-2View Details
N-Phenylhydroxylamine 90% CAS 100-65-2View Details
100-65-2 -
 N-Phenylhydroxylamine CAS 100-65-2View Details
N-Phenylhydroxylamine CAS 100-65-2View Details
100-65-2 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1