N-NITROSODI-N-PROPYLAMINE
Synonym(s):NDPA
- CAS NO.:621-64-7
- Empirical Formula: C6H14N2O
- Molecular Weight: 130.19
- MDL number: MFCD00013891
- EINECS: 210-698-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
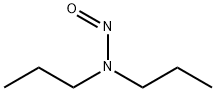
What is N-NITROSODI-N-PROPYLAMINE?
Chemical properties
Yellow Oil
Chemical properties
N-nitrosodi-N-propylamine is a yellow liquid.
The Uses of N-NITROSODI-N-PROPYLAMINE
N-Nitrosodi-n-propylamine is used in small quantities in laboratory research. It has no known commercial use (IARC 1978, ATSDR 1989, HSDB 2009).
The Uses of N-NITROSODI-N-PROPYLAMINE
Research chemical; impurity in herbicides treflan, isopropalin, and trifluralin; contaminant in wastewater from chemical factories and production of cheese and brandy and other liquors. N-nitrosamines are frequently produced during rubber processing and may be airborne in the workplace.
Definition
ChEBI: N-Nitrosodi-n-propylamine is a nitroso compound.
General Description
Clear to pale yellow liquid.
Reactivity Profile
N-NITROSODI-N-PROPYLAMINE is a nitrated amine derivative. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides.
Health Hazard
ACUTE/CHRONIC HAZARDS: Toxic.
Fire Hazard
Some may burn but none ignite readily. Containers may explode when heated. Some may be transported hot.
Safety Profile
Confirmed carcinogen with experimental carcinogenic, neoplastigenic, tumorigenic data. Moderately toxic by ingestion and subcutaneous routes. An experimental teratogen. Human mutation data reported. When heated to decomposition it emits toxic fumes of NOx. See also NITROSAMINES.
Potential Exposure
N-nitrosodi-N-propylamine is used in the manufacture of plastics, resins, rubber, and synthetic textiles. There is no evidence that N-nitrosodi-N-propylamine exists naturally in soil, air, food, or water. Small quantities of N-nitrosodi-N-propylamine are inadvertently produced during some manufacturing processes; as an impurity in some commercially available dinitroaniline based weed killers, and during the manufacture of some rubber products. However, according to Sax, some similar N-nitroso compounds are formed in the environment and absorbed from precursors in food, water, or air; from tobacco; and from naturally occurring compounds.
Carcinogenicity
N-Nitrosodi-n-propylamine is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
Environmental Fate
Chemical/Physical. N-Nitroso-n-propylamine will not hydrolyze because it does not contain a
hydrolyzable functional group (Kollig, 1993).
At influent concentrations of 1.0, 0.1, 0.01, and 0.001 mg/L, the GAC adsorption capacities were
24, 13, 7.4, and 4.0 mg/g, respectively (Dobbs and Cohen, 1980).
Shipping
UN3082 Environmentally hazardous substances, liquid, n.o.s., Hazard Class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Sensitive to UV light.
Waste Disposal
N-Nitrosodi-N-propylamine may be destroyed by high temperature incineration in an incinerator equipped with an nitrogen oxide scrubber. Chemical treatment methods may also be used to destroy N-nitrosodi-N-propylamine. These methods involve (a) denitrosation by reaction with 3% hydrobromic acid in glacial acetic acid; (b) oxidation by reaction with potassium permanganate-sulfuric acid; or (c) extraction of the nitrosamine from the waste using dichloromethane and subsequent reaction with triethyloxonium tetrafluoroborate (TOEF). Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
Properties of N-NITROSODI-N-PROPYLAMINE
| Boiling point: | 113 °C / 40mmHg |
| Density | 0.92 |
| refractive index | 1.4468 (589.3 nm 20℃) |
| Flash point: | 10 °C |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | Very soluble in ethanol and ether (quoted, Keith and Walters, 1992) |
| pka | -3.18±0.70(Predicted) |
| form | neat |
| color | Yellow to gold colored liquid |
| Water Solubility | 9,900 mg/L at 25 °C (Mirvish et al., 1976) |
| IARC | 2B (Vol. 17, Sup 7) 1987 |
| EPA Substance Registry System | N-Nitrosodi-n-propylamine (621-64-7) |
Safety information for N-NITROSODI-N-PROPYLAMINE
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H350:Carcinogenicity H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for N-NITROSODI-N-PROPYLAMINE
N-NITROSODI-N-PROPYLAMINE manufacturer
Svak Life Sciences
VIVAN Life Sciences Pvt Ltd
Synchemia Research Chemical
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 621-64-7 N-Nitroso di propylamine 99.70View Details
621-64-7 N-Nitroso di propylamine 99.70View Details
621-64-7 -
 N-Nitrosodipropylamine 621-64-7 98%View Details
N-Nitrosodipropylamine 621-64-7 98%View Details
621-64-7 -
 N-Nitrosodipropylamine CAS 621-64-7View Details
N-Nitrosodipropylamine CAS 621-64-7View Details
621-64-7 -
 621-64-7 98%View Details
621-64-7 98%View Details
621-64-7 -
 N-Nitrosodipropylamine 99%View Details
N-Nitrosodipropylamine 99%View Details
621-64-7 -
 N-Nitrosodipropylamine 98%View Details
N-Nitrosodipropylamine 98%View Details -
 N-Nitrosodi-n-propylamine CAS 621-64-7View Details
N-Nitrosodi-n-propylamine CAS 621-64-7View Details
621-64-7 -
 N-Nitrosodi-n-propylamine solution CAS 621-64-7View Details
N-Nitrosodi-n-propylamine solution CAS 621-64-7View Details
621-64-7