N-Nitrocarbamide
- CAS NO.:556-89-8
- Empirical Formula: CH3N3O3
- Molecular Weight: 105.05
- MDL number: MFCD00039768
- EINECS: 209-144-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 15:16:25
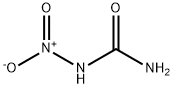
What is N-Nitrocarbamide?
Chemical properties
White, crystalline powder.Slightly soluble in water; soluble in alcohol, acetone, and acetic acid.
The Uses of N-Nitrocarbamide
Explosives.
General Description
A colorless to white crystalline powder solid. Mildly sensitive to heat and shock. An extremely powerful explosive. Decomposes to emit toxic nitrogen oxide fumes. May explode under exposure to intense heat or fire. Primary hazard is blast of an instantaneous explosion, not flying projectiles or fragments.
Air & Water Reactions
Hydrolysis occurs in water.
Reactivity Profile
Explosive mercury or silver salts are rather sensitive to heat and impact, while the pure material is much more insensitive. Organonitrate compounds, such as N-Nitrocarbamide, range from slight to strong oxidizing agents. If mixed with reducing agents, including hydrides, sulfides and nitrides, they may begin a vigorous reaction that culminates in a detonation. Nitroalkanes are milder oxidizing agents, but still react violently with reducing agents at higher temperature and pressures. Nitroalkanes react with inorganic bases to form explosive salts. The presence of metal oxides increases the thermal sensitivity of nitroalkanes.
Hazard
Severe explosion risk.
Health Hazard
Fire may produce irritating, corrosive and/or toxic gases.
Fire Hazard
MAY EXPLODE AND THROW FRAGMENTS 1600 meters (1 MILE) OR MORE IF FIRE REACHES CARGO.
Safety Profile
A very dangerous fire hazard when exposed to heat or flame. A severe explosion hazard when shocked or exposed to heat. Can react vigorously with oxidizing materials. It is a lugh explosive. Incompatible with mercuric and silver salts. When heated to decomposition it emits highly toxic fumes of NOx. See also EXPLOSIVES, HIGH; and NITRATES.
Purification Methods
Crystallise it from EtOH/pet ether. Dry it in vacuo ~50o. [Ingersoll & Arenendt Org Synth Coll Vol I 417 1941.]
Properties of N-Nitrocarbamide
| Melting point: | 158.5 ºC (DEC.) |
| Boiling point: | 197.01°C (rough estimate) |
| Density | 1.557±0.06 g/cm3(Predicted) |
| refractive index | 1.4451 (estimate) |
| pka | pK (20°) 2.15 |
| color | Crystals or leaflets or prisms from EtOH; orplatelets from EtOH/ligroin |
| NIST Chemistry Reference | Nitrourea(556-89-8) |
| EPA Substance Registry System | Urea, nitro- (556-89-8) |
Safety information for N-Nitrocarbamide
Computed Descriptors for N-Nitrocarbamide
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4