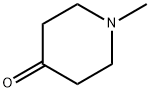
N-Methylpiperidine
Synonym(s):N-Methylpiperidine
- CAS NO.:626-67-5
- Empirical Formula: C6H13N
- Molecular Weight: 99.17
- MDL number: MFCD00006491
- EINECS: 210-959-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
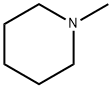
What is N-Methylpiperidine?
Description
This simple piperidine derivative has been obtained from Girgensohnia diptera Bge. It is optically inactive and furnishes a crystalline picrate, m.p. 15 I-2°C and a picrolonate, m.p. 226°C.
Chemical properties
CLEAR COLOURLESS LIQUID
The Uses of N-Methylpiperidine
Reactant for: ·sp3 C-H Bond activation with ruthenium(II) catalysts and C(3)-alkylation of cyclic amines ·One-pot synthesis of Z-cinnamic acids Reactant for synthesis of: ·Unsymmetrical ureas ·Antibacterial imidazolium, pyrrolidinium, and piperidinium salts ·C1-C16 segment of goniodomin A via palladium-catalyzed organostannane thioester coupling ·Multi-targeted inhibitors of insulin-like growth factor-1 receptor and members of ErbB-family receptor kinases
The Uses of N-Methylpiperidine
1-Methylpiperidine is a reactant for sp3 C-H Bond activation with ruthenium(II) catalysts and C(3)-alkylation of cyclic amines, One-pot synthesis of Z-cinnamic acids. It is used in the synthesis of Unsymmetrical ureas, Antibacterial imidazolium, pyrrolidinium, and piperidinium salts, C1-C16 segment of goniodomin A via palladium-catalyzed organostannane thioester coupling.
The Uses of N-Methylpiperidine
Reactant for:
- sp3 C-H Bond activation with ruthenium(II) catalysts and C(3)-alkylation of cyclic amines
- One-pot synthesis of Z-cinnamic acids
Reactant for synthesis of:
- Unsymmetrical ureas
- Antibacterial imidazolium, pyrrolidinium, and piperidinium salts
- C1-C16 segment of goniodomin A via palladium-catalyzed organostannane thioester coupling
- Multi-targeted inhibitors of insulin-like growth factor-1 receptor and members of ErbB-family receptor kinases
General Description
A colorless liquid with the odor of pepper. Density 0.816 g / cm3 and flash point 38°F. May irritate skin and eyes. Vapors heavier than air. Used as a solvent and to make other chemicals.
Air & Water Reactions
Highly flammable. Soluble in water.
Reactivity Profile
N-Methylpiperidine neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides.
Health Hazard
May cause toxic effects if inhaled or ingested/swallowed. Contact with substance may cause severe burns to skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
Flammable/combustible material. May be ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Flammability and Explosibility
Highly flammable
Safety Profile
Poison by intraperitoneal and subcutaneous routes. A very dangerous fire hazard when exposed to heat or flame. When heated to decomposition it emits toxic fumes of NOx.
References
Jurashevskii, Stepanov,J. Gen. Chem. USSR, 9,2203 (1939)
Properties of N-Methylpiperidine
| Melting point: | -50 °C |
| Boiling point: | 106-107 °C (lit.) |
| Density | 0.816 g/mL at 25 °C (lit.) |
| vapor pressure | 35.7hPa at 20℃ |
| refractive index | n |
| Flash point: | 38 °F |
| storage temp. | −20°C |
| solubility | Chloroform (Sparingly), Methanol (Slightly) |
| form | Liquid |
| pka | 10.08(at 25℃) |
| color | Clear colorless |
| PH | 12 (100g/l, H2O, 20℃) |
| explosive limit | 1.1-9.9%(V) |
| Water Solubility | miscible |
| BRN | 1073 |
| Dielectric constant | 2.5899999999999999 |
| CAS DataBase Reference | 626-67-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Piperidine, 1-methyl-(626-67-5) |
| EPA Substance Registry System | Piperidine, 1-methyl- (626-67-5) |
Safety information for N-Methylpiperidine
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H225:Flammable liquids H314:Skin corrosion/irritation H331:Acute toxicity,inhalation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. |
Computed Descriptors for N-Methylpiperidine
N-Methylpiperidine manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 1-Methyl piperidine 98%View Details
1-Methyl piperidine 98%View Details -
 626-67-5 98%View Details
626-67-5 98%View Details
626-67-5 -
 1-Methylpiperidine CAS 626-67-5View Details
1-Methylpiperidine CAS 626-67-5View Details
626-67-5 -
 1-Methylpiperidine CAS 626-67-5View Details
1-Methylpiperidine CAS 626-67-5View Details
626-67-5 -
 n-Methylpiperidine, 99% CAS 626-67-5View Details
n-Methylpiperidine, 99% CAS 626-67-5View Details
626-67-5 -
 N-Methylpiperidine CAS 626-67-5View Details
N-Methylpiperidine CAS 626-67-5View Details
626-67-5 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
