N-Methylhydroxylamine hydrochloride
- CAS NO.:4229-44-1
- Empirical Formula: CH6ClNO
- Molecular Weight: 83.52
- MDL number: MFCD00012597
- EINECS: 224-181-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-24 18:13:46
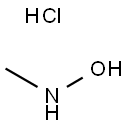
What is N-Methylhydroxylamine hydrochloride?
Chemical properties
The substance appears as white to almost white crystals or crystalline powder. It exhibits solubility in water.
The Uses of N-Methylhydroxylamine hydrochloride
N-Methylhydroxylamine hydrochloride is used as an inorganic catalyst used in the transamidation of primary amides with amines. It is employed in constructing hydroxamates, important functional groups for the complexation of iron.
Preparation
N-methylhydroxylamine hydrochloride (N-MHA) was synthesized by a high pressure catalytic hydrogenation method using noble metal Pd/C and Pt-Rh/C as the catalyst. During the synthesis process, the catalyst was easily poisoned and the products would be deeply reduced to methylamine. Therefore, the purity of N-MHA is low and the cost is high. An industrial electrolytic cell was designed for the electrochemical synthesis of N-methylhydroxylamine hydrochloride (N-MHA). Copper was used as the cathode, graphite as the anode, and a cation membrane as the separator. The results show that N-MHA with a high purity of 99% can be electrosynthesized directly from nitromethane in HCl solution.
Reactions
N-methylhydroxylamine hydrochloride was widely used in the 1,3-dipolar cycloaddition and N-methylation reaction. For example, it has been considered an extremely important intermediate in the synthesis of biological active azetidinone and isoxazole compounds such as piperylone, isopyrine, and muscimole.
References
[1] A. Gibson and S. Mirzazadeh. N-methylhydroxylamine inhibits and M&B 22948 potentiates relaxations of the mouse anococcygeus to non-adrenergic, non-cholinergic field stimulation and to nitrovasodilator drugs.British Journal of Pharmacology.1989, 96 637-644. DOI:10.1111/j.1476-5381.1989.tb11863.x
[2] J. R. Ochoa Gomez. Electrosynthesis of N-methylhydroxylamine.Journal of Applied Electrochemistry.1991, 21 331-334. DOI:10.1007/BF01020218
[3] Reacts with aldehydes and ketones to give nitrones, which undergo 1,3-dipolar addition reactions with alkenes. Cycloaddition to trimethylvinylsilane has been used in a 2-carbon extension of aldehydes to ɑ?-unsaturated aldehydes: J. Org. Chem., 49, 3421 (1984). DOI:10.1021/jo00192a047
[4] Intramolecular cycloaddition has been used as a route to bicyclic systems, e.g. from citronellal: Org. Synth. Coll., 6, 670 (1988).
[5] Reviews: 1,3-Dipolar cycloadditions of nitrones: Synthesis, 205 (1975). The [3+2] nitrone-olefin cycloaddition reaction: Org. React., 36, 1 (1988). Synthetic applications of nitrones: Org. Prep. Proced. Int., 17, 25 (1985).
[6] For conversion to, and reactions of, the N,O-bis(TMS) derivative, see: J. Chem. Soc., Perkin 1, 1823 (1989).
[7] GAN Y, WENKUI Z, HUANG H, et al. Industrial Synthesis of N-Methylhydroxylamine Hydrochloride by Electrochemical Reduction of Nitromethane[J]. Chinese Journal of Chemical Engineering, 2006, 14: 649-653. DOI:10.1016/S1004-9541(06)60129-8.
Properties of N-Methylhydroxylamine hydrochloride
| Melting point: | 86-88 °C(lit.) |
| Density | 1.36 g/cm3 |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | H2O: 0.1 g/mL, clear, very faintly yellow |
| form | solid |
| color | white |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| BRN | 3541409 |
| CAS DataBase Reference | 4229-44-1(CAS DataBase Reference) |
| EPA Substance Registry System | Methanamine, N-hydroxy-, hydrochloride (4229-44-1) |
Safety information for N-Methylhydroxylamine hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for N-Methylhydroxylamine hydrochloride
N-Methylhydroxylamine hydrochloride manufacturer
JSK Chemicals
ASM Organics
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 N-Methylhydroxylamine hydrochloride 99%View Details
N-Methylhydroxylamine hydrochloride 99%View Details -
 N-Methylhydroxylamine hydrochloride 99%View Details
N-Methylhydroxylamine hydrochloride 99%View Details
4229-44-1 -
 N-Methylhydroxylamine hydrochloride, 98% 99%View Details
N-Methylhydroxylamine hydrochloride, 98% 99%View Details
4229-44-1 -
 N-Methylhydroxylamine HCl 98% CAS 4229-44-1View Details
N-Methylhydroxylamine HCl 98% CAS 4229-44-1View Details
4229-44-1 -
 N-Methylhydroxylamine hydrochloride CAS 4229-44-1View Details
N-Methylhydroxylamine hydrochloride CAS 4229-44-1View Details
4229-44-1 -
 N-Methylhydroxylamine HCl 98.00% CAS 4229-44-1View Details
N-Methylhydroxylamine HCl 98.00% CAS 4229-44-1View Details
4229-44-1 -
 N-Methylhydroxylamine Hydrochloride CAS 4229-44-1View Details
N-Methylhydroxylamine Hydrochloride CAS 4229-44-1View Details
4229-44-1 -
 N-Methylhydroxylamine hydrochloride CAS 4229-44-1View Details
N-Methylhydroxylamine hydrochloride CAS 4229-44-1View Details
4229-44-1
