N-Methylbenzamide
- CAS NO.:613-93-4
- Empirical Formula: C8H9NO
- Molecular Weight: 135.16
- MDL number: MFCD00011642
- EINECS: 210-362-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-23 21:37:54
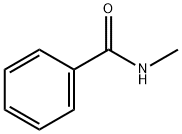
What is N-Methylbenzamide?
Chemical properties
N-methylbenzamide is an off-white crystalline solid.
The Uses of N-Methylbenzamide
N-Methylbenzamide is an important pesticide intermediate. It acts as a potent PDE10A (phosphodiesterase with a remarkable localization as the protein is abundant only in brain tissue) inhibitor.
Synthesis Reference(s)
Synthetic Communications, 7, p. 549, 1977 DOI: 10.1080/00397917709409275
Tetrahedron Letters, 30, p. 451, 1989 DOI: 10.1016/S0040-4039(00)95225-0
Air & Water Reactions
Insoluble in water.
Reactivity Profile
N-METHYLBENZAMIDE is an amide. Amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx).
Fire Hazard
Flash point data concerning N-METHYLBENZAMIDE are not available, however, N-METHYLBENZAMIDE is probably combustible.
Synthesis
Add benzoic acid (977 mg, 8 mmol), dry DCM (20 mL) and catalytic amount of DMF in a 100 mL round-bottom flask. Cool the reaction mixture to 0°C and stir for 5 minutes. Add (COCl)2 (0.89 mL, 1.3 equiv.) dropwise to the reaction mixture and stir at room temperature for 4 hours. Concentrate the resulting mixture under reduced pressure to obtain acid chloride. Add catalytic amount of 4-dimethylaminopyridine, dry DCM (15 mL), MeNH2 (2 (M) in THF) (5.19 mL, 1.3 equiv.) and Et3N (1.56 mL, 1.4 equiv.) to the mixture in a 100 mL round-bottom flask. Cool the reaction mixture to 0°C. Add acid chloride (1.0 equiv.) dropwise at 0°C. Stir the reaction mixture at room temperature for 12 hours. Add water (40 mL) and seperate the organic layer. Extract the aqueous layer with DCM (3 x 30 mL). Wash the combined organic layer with saturated aqueous NaHCO3 (30 mL) solution followed by water (30 mL). Dry the organic layer over Na2SO4 and concentrated under reduced pressure. Purify the crude mass by silica gel column chromatography (20% ethyl acetate in hexane as eluent) to obtain N-methylbenzamide. DOI: 10.1002/anie.202203539

Properties of N-Methylbenzamide
| Melting point: | 76-78 °C (lit.) |
| Boiling point: | 167 °C/11 mmHg (lit.) |
| Density | 1.1031 (rough estimate) |
| refractive index | 1.5589 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | ethanol: soluble50mg/mL, clear, yellow-green |
| form | Solid |
| pka | 15.00±0.46(Predicted) |
| color | White to off-white |
| Water Solubility | Insoluble in water. |
| InChI | InChI=1S/C8H9NO/c1-9-8(10)7-5-3-2-4-6-7/h2-6H,1H3,(H,9,10) |
| CAS DataBase Reference | 613-93-4(CAS DataBase Reference) |
| EPA Substance Registry System | N-Methylbenzamide (613-93-4) |
Safety information for N-Methylbenzamide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for N-Methylbenzamide
| InChIKey | NCCHARWOCKOHIH-UHFFFAOYSA-N |
| SMILES | C(NC)(=O)C1=CC=CC=C1 |
N-Methylbenzamide manufacturer
ALS India Life Sciences Pvt. Ltd
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 613-93-4 99%View Details
613-93-4 99%View Details
613-93-4 -
 N-Methylbenzamide CAS 613-93-4View Details
N-Methylbenzamide CAS 613-93-4View Details
613-93-4 -
 N-Methylbenzamide 95% CAS 613-93-4View Details
N-Methylbenzamide 95% CAS 613-93-4View Details
613-93-4 -
 613-93-4 N-Methylbenzamide 99.89%View Details
613-93-4 N-Methylbenzamide 99.89%View Details
613-93-4 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
