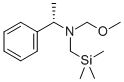
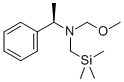
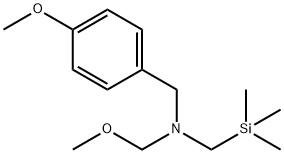
N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine
- CAS NO.:93102-05-7
- Empirical Formula: C13H23NOSi
- Molecular Weight: 237.41
- MDL number: MFCD00674005
- EINECS: 630-326-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-24 18:13:54
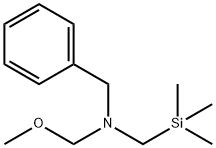
What is N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine?
Chemical properties
N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine is clear colorless to light yellow liquid
Physical properties
bp 77–80°C/0.5 mmHg.
The Uses of N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine
N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine is useful reagent in synthesizing N-benzyl substituted pyrrolidines by [3+2] cycloaddition to α,ßunsaturated esters.
The Uses of N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine
N-Benzyl-N-(methoxymethyl)-
N-trimethylsilylmethylamine (1) is a valuable reagent for in situ
generation of the N-benzyl azomethine ylide (2). It is generally
preferred over alternative silylmethylamine precursors6–8 because
of ease of handling and use. The ylide (2) is most conveniently
generated from (1) using a catalytic amount of trifluoroacetic acid
as described by Achiwa.Alternative catalysts include LiF,
TBAF,Me3SiOTf–CsF, or Me3SiI–CsF. Mechanistic studies
provide evidence that the reactive intermediate generated from
(1) with either CF3CO2H or F? is a 1,3-dipolar species. Reaction
of (2) with alkenes provides an efficient convergent route
to pyrrolidine derivatives. Alkynes afford 3-pyrrolines which can
be converted into pyrroles.The ylide (2) reacts most readily with
electron deficient alkenes and alkynes since this pairing results in
a narrow dipole HOMO–dipolarophile LUMO energy gap.Examples
of suitable dipolarophiles include unsaturated esters,
ketones, imides,nitriles,and sulfones. Cycloaddition occurs
with complete cis stereospecificity (eq 1) which is consistent
with a concerted mechanism. Dipolarophiles containing
an endocyclic double bond afford fused bicyclic pyrrolidines,
whereas substrates with an exocyclic double bond provide access
to spirocyclic systems.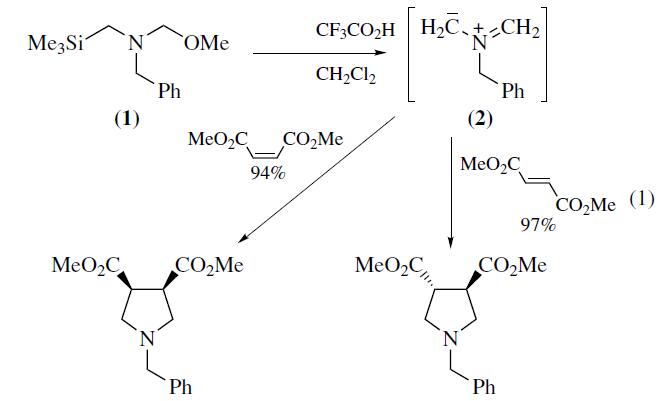
The Uses of N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine
N-Methoxymethyl-N-(trimethylsilylmethyl)benzylamine forms azomethine ylides which readily undergo [3+2] cycloaddition to α,?-unsaturated esters affording N-benzyl substituted pyrrolidines in good yields. It reacts with asymmetric 1,3-dipolar cycloadditions in the practical, large-scale synthesis of chiral pyrrolidines. It is used in the the synthesis of 3-carboxy-1-azabicyclo[2.2.1]heptane derivatives, an important class of physiologically active compounds.
What are the applications of Application
N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine is Reacted in asymmetric 1,3-dipolar cycloadditions in the practical, large-scale synthesis of chiral pyrrolidines
Preparation
most conveniently prepared by treatment of benzylamine with chloromethyltrimethylsilane followed by formaldehyde and methanol.Access to higher ether homologs is achieved by replacing methanol with the appropriate alcohol. An alternate procedure involves alkylation of lithium N-benzyltrimethylsilymethylamide with methoxymethyl chloride.
Properties of N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine
| Boiling point: | 76 °C0.3 mm Hg(lit.) |
| Density | 0.928 g/mL at 25 °C(lit.) |
| refractive index | n |
| Flash point: | 151 °F |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | Soluble in chloroform, ethyl acetate. |
| form | Liquid |
| pka | 7.29±0.50(Predicted) |
| Specific Gravity | 0.928 |
| color | Clear colorless to light yellow |
| Sensitive | Moisture & Light Sensitive |
| Hydrolytic Sensitivity | 2: reacts with aqueous acid |
| BRN | 4311216 |
| CAS DataBase Reference | 93102-05-7(CAS DataBase Reference) |
Safety information for N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine
| InChIKey | RPZAAFUKDPKTKP-UHFFFAOYSA-N |
N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine manufacturer
JSK Chemicals
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 93102-05-7 98%View Details
93102-05-7 98%View Details
93102-05-7 -
 N-Benzyl-N-(methoxymethyl)-N-trimethylsilylmethylamine 98%View Details
N-Benzyl-N-(methoxymethyl)-N-trimethylsilylmethylamine 98%View Details
93102-05-7 -
 N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine, 96% CAS 93102-05-7View Details
N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine, 96% CAS 93102-05-7View Details
93102-05-7 -
 N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine CAS 93102-05-7View Details
N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine CAS 93102-05-7View Details
93102-05-7 -
 N-Benzyl-N-(methoxymethyl)-N-trimethylsilylmethylamine CAS 93102-05-7View Details
N-Benzyl-N-(methoxymethyl)-N-trimethylsilylmethylamine CAS 93102-05-7View Details
93102-05-7 -
 N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine CAS 93102-05-7View Details
N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine CAS 93102-05-7View Details
93102-05-7 -
 N-Methoxymethyl-N-(trimethylsilylmethyl)benzylamine CAS 93102-05-7View Details
N-Methoxymethyl-N-(trimethylsilylmethyl)benzylamine CAS 93102-05-7View Details
93102-05-7 -
 N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine CAS 93102-05-7View Details
N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine CAS 93102-05-7View Details
93102-05-7
