N-Bromosuccinimide
Synonym(s):NBS;N-Bromosuccinimide;NBN;Cell cycle regulatory protein P95;NIBRIN
- CAS NO.:128-08-5
- Empirical Formula: C4H4BrNO2
- Molecular Weight: 177.98
- MDL number: MFCD00005510
- EINECS: 204-877-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-01 18:33:23
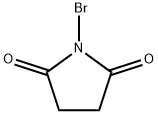
What is N-Bromosuccinimide?
Description
N-Bromosuccinimide (NBS) is a reagent typically used in radical substitution and electrophilic addition reactions. In pure form it exists as a white solid but over time it will decompose to give off bromine which will give it a slight yellow/brown color.
Chemical properties
white to light yellow crystalline powder
The Uses of N-Bromosuccinimide
N-Bromosuccinimide is a brominated succinimide used as a chemical reagent in radical substitution and electrophilic addition reactions in organic synthesis.
The Uses of N-Bromosuccinimide

To a solution of the SM (80 g, 0.27 mol) in CHCl3 (1 L) at 10-15 C was added dropwise a solution of Br2 (16 mL, 0.31 mol) in CHCl3 (100 mL). The reaction was stirred at 12 C for 10 min, then washed sequentially with cooled sat aq NaHCO3 (2 x 500 mL) and sat aq brine (400 mL), dried, and concentrated in vacuo. Crystallization of the residue from EtOAc provided the product as a yellow solid. [90 g, 88%]
What are the applications of Application
N-Bromosuccinimide is used in radical substitution and electrophilic addition reactions.
It can be used as a reagent:
In the Wohl-Ziegler reaction (bromination at allylic positions via a radical pathway).
To synthesize benzils and aliphatic 1,2-diketones from hydrobenzoins and 1,2-diols in the presence of CCl4 as a solvent.
To prepare tricyclic azepino[4,5-b]indoles from indole-β-enaminoesters or β-enaminones via Pictet–Spengler cyclization.
To synthesize acylsilanes via oxidative hydrolysis of 2-silyl-1,3-dithianes.
Versatile brominating agent. For the oxidation of tryptophan though tyrosine, histidine and methionine residues may be oxidized to a lesser extent. Also used for the modification of ribosomal sulfhydryl groups.
Definition
ChEBI: N-bromosuccinimide is a five-membered cyclic dicarboximide compound having a bromo substituent on the nitrogen atom. It has a role as a reagent. It is a dicarboximide, a pyrrolidinone and an organobromine compound. It is functionally related to a succinimide.
Preparation
N-Bromosuccinimide is prepared by addition of bromine to a cold aqueous solution of succinimide or by reaction of succinimide with NaBrO2 in the presence of HBr.
1.62mol (160g) succinimide is dissolved in a mixture of 1.60mol (64g) NaOH, 300g crushed ice and 400ml water. Cool the mixture in an ice bath, and add 85ml (1.65 mol, 264g) Br2 at once while stirring violently. Stir for two more minutes, then filter the precipitated product and wash with ice water. Dry in a desiccator. Yield 75-81%.
Don't clean up NBS too much, the stinky yellow stuff still containing a bit of Br2 works best. N-Bromosuccinimide is a brominating agent that replaces hydrogen atoms in benzylic or allylic positions. It is used in the oxidation of secondary alcohols to ketones and in controlled low-energy brominations.
Flammability and Explosibility
Non flammable
Safety Profile
Poison by intraperitoneal route. An irritating poison to skin, eyes, and mucous membranes. Reacts explosively with adme, dtallyl sulfide, and hydrazine hydrate. Explosive reaction with propiononitrile after heating to 105℃ for 24 hours. Violent reaction with dtbenzoyl peroxide + 4-tok acid. When heated to decomposition it emits toxic fumes of Brand NOx. See also BROMIDES and NITROGEN MONOXIDE.
Definition
N-bromosuccinimide is a five-membered cyclic dicarboximide compound having a bromo substituent on the nitrogen atom. It has a role as a reagent and is functionally related to a succinimide. It is a dicarboximide, a pyrrolidinone, and an organobromine compound.
Purification Methods
N-Bromosuccinimide (30g) is purified by dissolving rapidly in 300mL of boiling water and filtering through a fluted filter paper into a flask immersed in an ice bath, and left for 2hours. The crystals are filtered off, washed thoroughly with ca 100mL of ice-cold water and drained on a Büchner funnel before drying under vacuun over P2O5 or CaCl2 [Dauben & McCoy J Am Chem Soc 81 4863 1959]. This brominating agent has also been crystallised from acetic acid or water (10 parts), washed in water and dried in vacuo [Wilcox et al. J Am Chem Soc 108 7693 1986, Shell et al. J Am Chem Soc 108 121 1986, Phillips & Cohen J Am Chem Soc 108 2013 1986, Beilstein 21/9 V 543.]
Properties of N-Bromosuccinimide
| Melting point: | 175-180 °C (dec.)(lit.) |
| Boiling point: | 221.4°C (rough estimate) |
| Density | 2.098 |
| vapor pressure | 14.8 hPa (20 °C) |
| refractive index | 1.6060 (estimate) |
| storage temp. | Store at +2°C to +8°C. |
| solubility | 14.8g/l (decomposition) |
| form | Crystalline Powder |
| appearance | White solid |
| pka | -2.78±0.20(Predicted) |
| color | White to light yellow |
| Odor | characteristic odour of bromine |
| Water Solubility | Soluble in acetone, tetrahydrofuran, dimethyl formamide, dimethyl sulfoxide and acetonitrile. Slightly soluble in water and acetic acid. Insoluble in ether, hexane and carbon tetrachloride. |
| Sensitive | Moisture Sensitive |
| Merck | 14,1438 |
| BRN | 113916 |
| Stability: | Stable. Incompatible with strong oxidizing agents, halogenated hydrocarbons. |
| CAS DataBase Reference | 128-08-5(CAS DataBase Reference) |
| NIST Chemistry Reference | 2,5-Pyrrolidinedione, 1-bromo-(128-08-5) |
| EPA Substance Registry System | N-Bromosuccinimide (128-08-5) |
Safety information for N-Bromosuccinimide
| Signal word | Warning |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H290:Corrosive to Metals H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H341:Germ cell mutagenicity H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for N-Bromosuccinimide
| InChIKey | PCLIMKBDDGJMGD-UHFFFAOYSA-N |
N-Bromosuccinimide manufacturer
NRS Chemicals LLP
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 N-Bromosuccinimide supplierView Details
N-Bromosuccinimide supplierView Details
128-08-5 -
 N-Bromosuccinimide 99%View Details
N-Bromosuccinimide 99%View Details -
 N-Bromo Succinimide 99%View Details
N-Bromo Succinimide 99%View Details -
 N-Bromosuccinimide pure CAS 128-08-5View Details
N-Bromosuccinimide pure CAS 128-08-5View Details
128-08-5 -
 N-Bromosuccinimide extrapure AR CAS 128-08-5View Details
N-Bromosuccinimide extrapure AR CAS 128-08-5View Details
128-08-5 -
 N BromosuccinimideView Details
N BromosuccinimideView Details
128-08-5 -
 N Bromosuccinimide ChemicalView Details
N Bromosuccinimide ChemicalView Details
128-08-5 -
 Powder N-Bromosuccinimide, Packaging Type: Bag, Packaging Size: 25 kgView Details
Powder N-Bromosuccinimide, Packaging Type: Bag, Packaging Size: 25 kgView Details
128-08-5
