Linalyl acetate
Synonym(s):3,7-Dimethyl-1,6-octadien-3-yl acetate;Bergamol;Linalyl acetate
- CAS NO.:115-95-7
- Empirical Formula: C12H20O2
- Molecular Weight: 196.29
- MDL number: MFCD00008907
- EINECS: 204-116-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-20 21:15:22
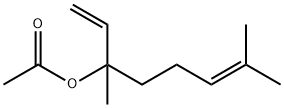
What is Linalyl acetate?
Chemical properties
Linalyl Acetate occurs as its isomer as the main component of lavender oil (30–60%, depending on the origin of the oil), of lavandin oil (25–50%, depending on the species), and of bergamot oil (30–45%). It has also been found in clary sage oil (up to 75%) and in a small amount in many other essential oils. Racemic linalyl acetate is a colorless liquid with a distinct bergamot–lavender odor.

Linalyl acetate is used extensively in perfumery. It is an excellent fragrance material for, among others, bergamot, lilac, lavender, linden, neroli, ylang-ylang, and fantasy notes (particularly chypre). Smaller amounts are used in other citrus products. Since linalyl acetate is fairly stable toward alkali, it can also be employed in soaps and detergents.
Chemical properties
Linalyl acetate has a characteristic bergamot–lavender odor and persistent sweet, acrid taste.
Occurrence
Reported found in the essential oils of bergamot, lavender, clary sage and lavandin; also identified among the constituents of the essential oils of Salvia officinalis, petitgrain, sassafras, neroli, lemon, Italian lime, jasmine, Mentha citrata, Mentha aquatica, Thymus mastichina, etc; also reported in abundant quantities in the essential oil from flowers, leaves and stems of Tagetes patula, in the distillate from leaves of Citrus aurantifolia from India, and in the essential oil of Mentha arvensis. Also reported found in citrus peel oils and juices, berries, peach, celery, tomato, cinnamon, clove, nutmeg, pepper, thymus, grape wines, avocado, mushroom, marjoram, mango, cardamom, coriander, gin, origanum, lovage, laurel, myrtle leaf, rosemary, sage and mastic gum oil.
The Uses of Linalyl acetate
Linalyl Acetate ermentative production of medium or short chain length alcohol, esters and/or glucosides by metabolically engineered microorganism.
The Uses of Linalyl acetate
In perfumery.
Preparation
Linalyl acetate is synthesized by two methods:
1) Esterification of linalool requires special reaction conditions since it tends to
undergo dehydration and cyclization as it is an unsaturated tertiary alcohol.
These reactions can be avoided as follows: esterification with ketene in the
presence of an acidic esterification catalyst below 30 °C results in the formation
of linalyl acetate without any by-products. Esterification can be achieved in good yield, with boiling acetic anhydride, whereby the acetic
acid is distilled off as it is formed; a large excess of acetic anhydride must
be maintained by continuous addition of anhydride to the still vessel.
Highly pure linalyl acetate can be obtained by transesterification of tert-butyl
acetate with linalool in the presence of sodium methylate and by continuous
removal of the tert-butanol formed in the process.
2) Dehydrolinalool, obtained by ethynylation of 6-methyl-5-hepten-2-one, can
be converted into dehydrolinalyl acetatewith acetic anhydride in the presence
of an acidic esterification catalyst. Partial hydrogenation of the triple bond
to linalyl acetate can be carried out with, for example, palladium catalysts
deactivated with lead.
Definition
ChEBI: 3,7-dimethylocta-1,6-dien-3-yl acetate is a monoterpenoid that is the acetate ester of linalool. It forms a principal component of the essential oils from bergamot and lavender. It is an acetate ester and a monoterpenoid. It is functionally related to a linalool.
Aroma threshold values
Detection: 1 ppm
Taste threshold values
Taste characteristics at 5 ppm: floral, green, waxy, terpy, citrus, herbal and spicy nuances.
General Description
Linalyl acetate is a monoterpene ester mainly found in lavandula essential oil. It is used as a flavoring agent in food industries, as well as a preservative additive in cosmetics and fragrances such as soaps, colognes, perfumes, and skin lotions.
Contact allergens
Structurally close to linalool, linalyl acetate is the main component of lavender oil and is commonly used in fragrances and toiletries, and in household cleaners and detergents as well. By autoxidation, it leads mainly to hydroperoxides, with a high sensitizing potent.
Synthesis
Normally prepared by direct acetylation of linalool; another method starts from myrcene hydrochloride, anhydrous sodium acetate and acetate anhydride in the presence of a catalyst; all synthetic methods tend to avoid the simultaneous formation (because of isomerization) of terpenyl and geranyl acetate.
Properties of Linalyl acetate
| Melting point: | 85°C |
| Boiling point: | 220 °C(lit.) |
| Density | 0.901 g/mL at 25 °C(lit.) |
| vapor density | 6.8 (vs air) |
| vapor pressure | 0.1 mm Hg ( 20 °C) |
| refractive index | n |
| FEMA | 2636 | LINALYL ACETATE |
| Flash point: | 194 °F |
| storage temp. | -20°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Liquid |
| color | Clear colorless |
| Odor | at 100.00 %. sweet green citrus bergamot lavender woody |
| Water Solubility | 499.8mg/L(25 ºC) |
| Merck | 14,5496 |
| JECFA Number | 359 |
| BRN | 1724500 |
| CAS DataBase Reference | 115-95-7(CAS DataBase Reference) |
| NIST Chemistry Reference | 1,6-Octadien-3-ol, 3,7-dimethyl-, acetate(115-95-7) |
| EPA Substance Registry System | Linalyl acetate (115-95-7) |
Safety information for Linalyl acetate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P272:Contaminated work clothing should not be allowed out of the workplace. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Linalyl acetate
| InChIKey | UWKAYLJWKGQEPM-LBPRGKRZSA-N |
Linalyl acetate manufacturer
Vikas Aromatics
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Linalyl acetate 99%View Details
Linalyl acetate 99%View Details -
 Linalyl acetate 98.00% CAS 115-95-7View Details
Linalyl acetate 98.00% CAS 115-95-7View Details
115-95-7 -
 Linalyl AcetateView Details
Linalyl AcetateView Details
115-95-7 -
 LINALYL ACETATEView Details
LINALYL ACETATEView Details
115-95-7 -
 Linalyl Acetate Chemical, Purity: 99%, Packaging Size: 1-170 KgView Details
Linalyl Acetate Chemical, Purity: 99%, Packaging Size: 1-170 KgView Details
115-95-7 -
 LINALYL ACETATEView Details
LINALYL ACETATEView Details
115-95-7 -
 1kg Linalyl Acetate, Grade: PerfumeView Details
1kg Linalyl Acetate, Grade: PerfumeView Details
115-95-7 -
 Linalyl Acetate, For Fragrances, Pharmaceuticals, Grade Standard: IndustrialView Details
Linalyl Acetate, For Fragrances, Pharmaceuticals, Grade Standard: IndustrialView Details
115-95-7
