Boc-Lys-OH
Synonym(s):Nα-(tert-Butoxycarbonyl)-L -lysine;Nα-Boc-L -lysine;Boc-Lys-OH;N-α-t.-Boc-L-lysine
- CAS NO.:13734-28-6
- Empirical Formula: C11H22N2O4
- Molecular Weight: 246.3
- MDL number: MFCD00038203
- EINECS: 237-303-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
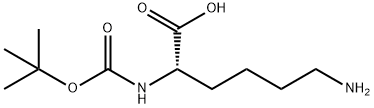
What is Boc-Lys-OH?
Chemical properties
white to off-white powder
The Uses of Boc-Lys-OH
N-Boc-L-lysine is a protected analogue of a major amino acid participating in the binding of AcH to proteins. N-Boc-L-lysine as well as other t-butoxycarbonyl (Boc) amino acids can be converted to the ir respective amino acids via cleavage of the urethane bond.
The Uses of Boc-Lys-OH
N-Boc-L-lysine is a protected analogue of a major amino acid participating in the binding of AcH to proteins. N-Boc-L-lysine as well as other t-butoxycarbonyl (Boc) amino acids can be converted to their respective amino acids via cleavage of the urethane bond.
The Uses of Boc-Lys-OH
Boc-Lys-OH (Nα-Boc-L-lysine) can be used as a building block to synthesize:
A heterotrifunctional peptide-based linker molecule applicable as a bio-labeling reagent.
Lysine derivatives of azamacrocycle and anthraquinone.
Boc-Lys(Bn4-DTPA)-OH, a precursor to synthesize diethylene triamine pentaacetic acid (DTPA) containing peptides.
A ferrocene-amino acid conjugate which is used in developing chemical warfare agent (CWA) sensor.
What are the applications of Application
Boc-Lys-OH is an N-Boc protected lysine derivative
Production Methods
L-lysine was added to acetone and Et3N under stirring at 25 °C. After that, di-tert-butyl dicarbonate was added. After 4.5 h, decompression evaporates acetone, and the water layer is extracted with ethyl ether 3 times. The dilute HC1 was added to the aqueous layer to adjust pH 2-3. The crude product was purified to obtain Boc-Lys-OH.
reaction suitability
Boc solid-phase peptide synthesis
Properties of Boc-Lys-OH
| Melting point: | ~205 °C (dec.)(lit.) |
| Boiling point: | 389.3°C (rough estimate) |
| alpha | 22 º (c=2, CH3OH) |
| Density | 1.1313 (rough estimate) |
| refractive index | 21.5 ° (C=2, MeOH) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | Acetic Acid (Slightly), Methanol (Slightly, Sonicated), Water (Slightly, Heated, |
| form | Powder |
| pka | 3.92±0.21(Predicted) |
| color | White |
| optical activity | [α]20/D +4.6±0.5°, c = 2% in H2O |
| Water Solubility | Soluble in water. Slightly soluble in methanol. |
| BRN | 4252546 |
| InChI | InChI=1S/C11H22N2O4/c1-11(2,3)17-10(16)13-8(9(14)15)6-4-5-7-12/h8H,4-7,12H2,1-3H3,(H,13,16)(H,14,15)/t8-/m0/s1 |
| CAS DataBase Reference | 13734-28-6(CAS DataBase Reference) |
Safety information for Boc-Lys-OH
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for Boc-Lys-OH
| InChIKey | DQUHYEDEGRNAFO-QMMMGPOBSA-N |
| SMILES | C(O)(=O)[C@H](CCCCN)NC(OC(C)(C)C)=O |
Boc-Lys-OH manufacturer
New Products
3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Iodophenylacetic acid Cyclohexane, (2-propynyloxy)- (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine Pivalic anhydride,98% Phenylmethanesulfonyl fluoride, 98% Glyoxylic acid solution, 50% in water tert-Butyl glycinate,97% 4-Ethoxybenzoic acid, 99% Sodium 1-octanesulfonate monohydrate 7-Ethyl Tryptophol 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl N, N-Carbonyldiimidazole (CDI) 5-Cyanophthalide 10-Methoxy-5H-dibenz[b,f]azepine 3-Methoxybenzonitrile Dibenzoyl Peroxide 4-Methoxybenzonitrile Titanium Dioxide Chloral PentachlorobenzonitrileRelated products of tetrahydrofuran


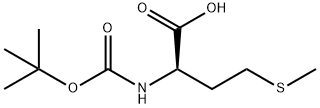
You may like
-
 13734-28-6 N-alpha-(tert-Butoxycarbonyl)-L-lysine 98%View Details
13734-28-6 N-alpha-(tert-Butoxycarbonyl)-L-lysine 98%View Details
13734-28-6 -
 13734-28-6 98%View Details
13734-28-6 98%View Details
13734-28-6 -
 Boc-L-Lys-OH 98% CAS 13734-28-6View Details
Boc-L-Lys-OH 98% CAS 13734-28-6View Details
13734-28-6 -
 Boc-l-lysine CAS 13734-28-6View Details
Boc-l-lysine CAS 13734-28-6View Details
13734-28-6 -
 Nα-(tert-Butoxycarbonyl)-L-lysine CAS 13734-28-6View Details
Nα-(tert-Butoxycarbonyl)-L-lysine CAS 13734-28-6View Details
13734-28-6 -
 Boc-Lys-OH CAS 13734-28-6View Details
Boc-Lys-OH CAS 13734-28-6View Details
13734-28-6 -
 Boc-Lys-OH CAS 13734-28-6View Details
Boc-Lys-OH CAS 13734-28-6View Details
13734-28-6 -
 Boc-Lys-OH CAS 13734-28-6View Details
Boc-Lys-OH CAS 13734-28-6View Details
13734-28-6
