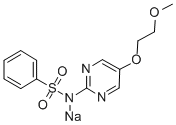
N-[5-(2-methoxyethoxy)pyrimidin-2-yl]benzenesulfonamide
- CAS NO.:339-44-6
- Empirical Formula: C13H15N3O4S
- Molecular Weight: 309.3409
- MDL number: MFCD00865503
- EINECS: 2064265
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-28 23:16:16
![N-[5-(2-methoxyethoxy)pyrimidin-2-yl]benzenesulfonamide Structural](https://img.chemicalbook.in/CAS/GIF/339-44-6.gif)
What is N-[5-(2-methoxyethoxy)pyrimidin-2-yl]benzenesulfonamide?
Absorption
Rapidly and completely absorbed following oral administration.
Toxicity
Severe hypoglycemic reactions with coma, seizure, or other neurological impairment.
Originator
Redul,Bayer/Schering,W. Germany,1964
The Uses of N-[5-(2-methoxyethoxy)pyrimidin-2-yl]benzenesulfonamide
Glymidine is used in biological activities to predict human intestinal absorption in drug discovery.
Background
Glycodiazine is used with diet to lower blood glucose by increasing the secretion of insulin from pancreas and increasing the sensitivity of peripheral tissues to insulin. The mechanism of action of glycodiazine in lowering blood glucose appears to be dependent on stimulating the release of insulin from functioning pancreatic beta cells, and increasing sensitivity of peripheral tissues to insulin. Glycodiazine likely binds to ATP-sensitive potassium channel receptors on the pancreatic cell surface, reducing potassium conductance and causing depolarization of the membrane. Membrane depolarization stimulates calcium ion influx through voltage-sensitive calcium channels. This increase in intracellular calcium ion concentration induces the secretion of insulin. It is used for the concomitant use with insulin for the treatment of noninsulin-dependent (type 2) diabetes mellitus.
Indications
Glycodiazine is used concomitantly with insulin for the treatment of noninsulin-dependent (type 2) diabetes mellitus.
Definition
ChEBI: Glymidine is a sulfonamide that is N-(pyrimidin-2-yl)benzenesulfonamide which is substituted at position 5 of the pyrimidine ring by a 2-methoxyethoxy group. It is a hypoglycemic drug used for the treatment of diabetes mellitus. It has a role as a hypoglycemic agent. It is a member of pyrimidines, a sulfonamide and a diether. It is a conjugate acid of a glymidine(1-).
Manufacturing Process
210 g phosphorus pentachloride are gradually added to 252 g
methoxyethoxyacetaldehyde-di-methoxyethylacetal with agitation. The
mixture is externally cooled with ice to hold the reaction temperature below
25°C. Moisture is carefully excluded. After addition of the condensation agent
is completed, the reaction mixture is further agitated at room temperature for
30 minutes. 225 ml dimethylformamide are then added drop by drop while
the reaction temperature is held at 20°C to 25°C by external cooling of the
reaction vessel with ice. When the dimethylformamide has been added, the
temperature is raised to 60°C, and this temperature is maintained for 70
minutes.
The temperature is again lowered to 20°C to 25°C and maintained at this
value by cooling with ice while 500 ml methanol are added drop by drop. The
resulting solution is admixed drop by drop to a suspension of 240 g powdered
caustic soda in 800 ml methanol at 20°C to 25°C. After mixing is completed,
stirring is continued for 30 minutes at room temperature. The solution now
contains inorganic salts and β-dimethylamino-α-methoxyethoxyacrolein.
200 g guanidine nitrate and thereafter 70 g sodium hydroxide are added to
the solution. The methanol is evaporated with agitation. The residue is
dissolved in 1.5 liters water and is repeatedly extracted with chloroform. The
combined chloroform extracts are evaporated to dryness, and the residue is
recrystallized from carbon tetrachloride. 80 g of 2-amino-5-
methoxyethoxypyrimidine of MP 80°C to 81°C are obtained.
This material is then dissolved in pyridine. Benzenesulfonylchloride is added
and the resulting mixture is heated two hours to 60°C. It is then poured into
300 ml water. The precipitate formed thereby is filtered off and dissolved in
dilute ammonium hydroxide. The solution is purified with charcoal, and
filtered. The filtrate is acidifed with acetic acid to give glymidine.
62 g 2-benzenesulfonamido-5-methoxyethoxypyrimidine are dissolved jointly
with 8 g sodium hydroxide in 250 ml ethanol. The solution is evaporated to
dryness, and the residue is suspended in 300 ml acetone. The sodium salt of
2-benzenesulfonamido-5-methoxyethoxypyrimidine may be filtered off,
washed with acetone, and dried. The yield of glymidine sodium is about 60 g,
the MP 220°C to 223°C.
Therapeutic Function
Antidiabetic
Pharmacokinetics
Glycodiazine is used with diet to lower blood glucose by increasing the secretion of insulin from pancreas and increasing the sensitivity of peripheral tissues to insulin.
Metabolism
Not Available
Properties of N-[5-(2-methoxyethoxy)pyrimidin-2-yl]benzenesulfonamide
| Melting point: | 152-154° |
| storage temp. | Refrigerator |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly) |
| form | Solid |
| color | Light Beige |
Safety information for N-[5-(2-methoxyethoxy)pyrimidin-2-yl]benzenesulfonamide
Computed Descriptors for N-[5-(2-methoxyethoxy)pyrimidin-2-yl]benzenesulfonamide
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium 3-Bromopyrazole (3aR,4R,5R,6aS)-hexahydro-5-Triethyl silyloxy-4-((E)-3-oxo-5-phenylpent-1- enyl)cyclopenta[b]furan-2-one. 1-Chlorocarbonyl-4-piperidinopiperidine 1-Bromo-4-phenyl-2-Butanone 4-Amino-2-fluoro-N-methylbenzamide 1,1'-Carbonyldiimidazole SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTIONRelated products of tetrahydrofuran
![N-[5-(2-methoxyethoxy)pyrimidin-2-yl]benzenesulfonamide](https://img.chemicalbook.in/CAS/GIF/339-44-6.gif)
You may like
-
![Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%View Details
Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%View Details
54094-19-8 -
 85-81-4 99%View Details
85-81-4 99%View Details
85-81-4 -
 Cyclopentane carboxxylic acid 3400-45-1 99%View Details
Cyclopentane carboxxylic acid 3400-45-1 99%View Details
3400-45-1 -
![208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%View Details
208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%View Details
208111-98-2 -
 2033-24-1 99%View Details
2033-24-1 99%View Details
2033-24-1 -
 Meldrums acid 2033-24-1 99%View Details
Meldrums acid 2033-24-1 99%View Details
2033-24-1 -
 Cyaclopentane carboxylic acid 99%View Details
Cyaclopentane carboxylic acid 99%View Details
3400-45-1 -
 2-Aminopyridine 504-29-0 99%View Details
2-Aminopyridine 504-29-0 99%View Details
504-29-0