MYCOPHENOLATE SODIUM
- CAS NO.:37415-62-6
- Empirical Formula: C17H21NaO6
- Molecular Weight: 344.34
- MDL number: MFCD01723176
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-28 16:00:23

What is MYCOPHENOLATE SODIUM?
Description
Mycophenolate sodium, an immunosuppressive agent, was launched in Switzerland as an oral treatment in combination with Neoral? and corticosteroids for the prevention of acute transplant rejection in adult patients receiving allogeneic renal transplantation. In contrast to the previously marketed product mycophenolate mofetil (MMF, CellCept?), which is a prodrug and must be converted to mycophenolic acid (MPA) in vivo, Myfortic? contains MPA itself as the active ingredient. Myfortic? was designed to enhance the therapeutic efficacy of MPA through increased tolerability relative to systemic exposure. Unlike MMF, which is absorbed in the stomach, the enteric-coated formulation of MPA sodium is mainly absorbed in the small intestine, thus protecting the upper GI tract from the side effects of MPA. MPA is an inhibitor of inosine monophosphate dehydrogenase (IMDPH), a vital enzyme in the de novo pathway of purine biosynthesis. Proliferating lymphocytes rely principally on this pathway for purine production,thus rendering them succeptible to depletion of purine bases by MPA. Inhibition of IMPDH by MPA results in the inhibition of both T- and B-lymphocyte proliferation upon antigen challenge and facilitates the prevention of acute graft rejection. Following oral administration of MPA sodium, the tmax of MPA is 1.5–2 h, with a mean absolute bioavailability of 71% and a mean half-life of 11.7 h. MPA is primarily metabolized in the liver by glucuronidation and excreted mainly in the urine as the metabolite. The recommended dosage regimen of MPA sodium is 720 mg twice daily, which provides equimolar amounts of MPA compared with MMF 1000 mg twice daily. In two major clinical trials in 748 patients, Myfortic? was demonstrated to be a highly potent and well-tolerated immunosuppressant for new renal transplant patients. A trend was seen towards fewer dose reductions due to GI intolerability and less serious infections relative to other MPA drugs. The most common adverse events associated with MPA treatment are diarrhea and leukopenia.
Originator
Novartis (Switzerland)
The Uses of MYCOPHENOLATE SODIUM
Immunosuppressant
The Uses of MYCOPHENOLATE SODIUM
Mycophenolic Acid Monosodium Salt in its enteric-coated form is used as an immunosuppressive agent in organ transplantation and autoimmune diseases.Environmental contaminants; Food contaminants
Definition
ChEBI: An organic sodium salt that is the sodium salt of mycophenolic acid. An immunosuppressant, it is widely used to prevent tissue rejection following organ transplants and for the treatment of certain autoimmune diseases.
brand name
Myfortic
Synthesis
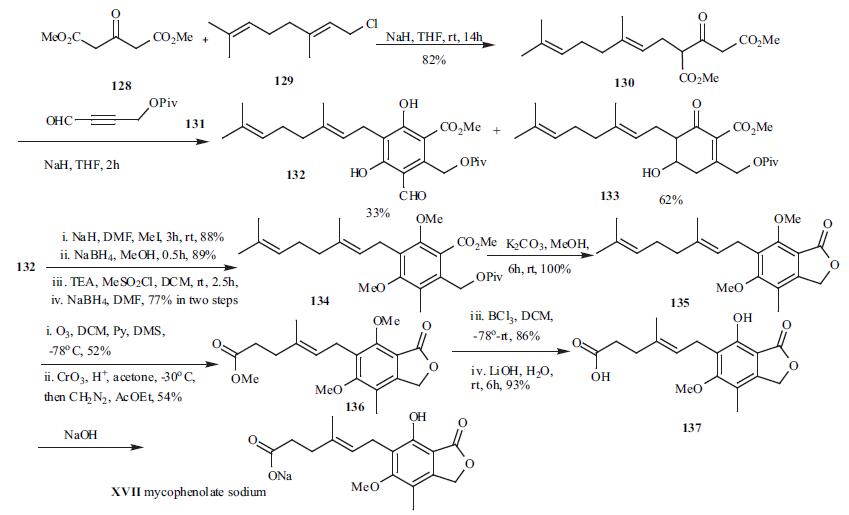
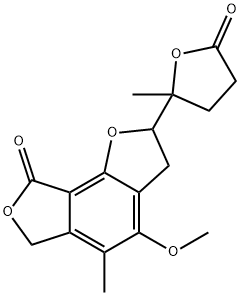
Mycophenolic acid (137) was originally synthesized by Birch and Wright and has been the subject of several total and formal syntheses. The large production in industry is done via fermentation. A concise synthesis of mycophenolic acid published recently is depicted in Scheme 17. Reaction of dimethyl 1,3-acetonedicarboxylate (128) with commercially available geranyl chloride (129) in the presence of NaH gave ketoester 130 in 82% yield. Treatment of ketoester 130 with 4-(pivolyloxy)-2-butynal (131) in the presence of NaH provided resorcinol 132 in a single step with all substituents in place in 33% yield along with two more compounds represented by 133 (62%). Resorcinol 132 was transformed into 134 via a four step sequences: methylation with NaH and MeI in dry DMF, reduction of the formyl group with NaBH4, mesylation of the resulting alcohol and subsequent reduction of the mesylate. The preparation of phthalide 135 was affected in quantitative yield on treatment of 134 with K2CO3 in dry MeOH. Selective ozonolysis of compound 135, followed by Jones oxidation and esterification afforded ester 136. Demethylation with BCl3 in DCM followed by hydrolysis of the ester function gave the mycophenolic acid (137). The mycophenolic acid was then converted to its sodium salt XVII (no conditions and yield available£?.
Safety information for MYCOPHENOLATE SODIUM
MYCOPHENOLATE SODIUM manufacturer
Sumar biotech LLP
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

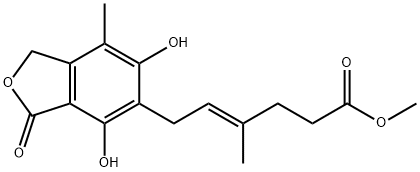
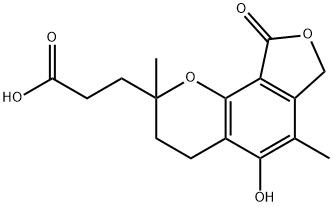

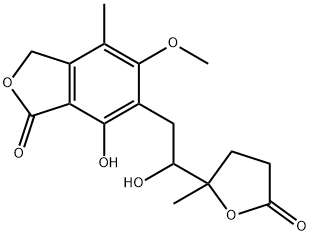
You may like
-
 37415-62-6 Mycophenolate sodium 98%View Details
37415-62-6 Mycophenolate sodium 98%View Details
37415-62-6 -
 37415-62-6 99%View Details
37415-62-6 99%View Details
37415-62-6 -
 Mycophenolate sodium 98%View Details
Mycophenolate sodium 98%View Details
37415-62-6 -
 Mycophenolate sodium 37415-62-6 98%View Details
Mycophenolate sodium 37415-62-6 98%View Details
37415-62-6 -
 Mycophenolate sodium 99%View Details
Mycophenolate sodium 99%View Details
37415-62-6 -
 Mycophenolate sodium 37415-62-6 98%View Details
Mycophenolate sodium 37415-62-6 98%View Details
37415-62-6 -
 Mycophenolate sodium 98% (HPLC) CAS 37415-62-6View Details
Mycophenolate sodium 98% (HPLC) CAS 37415-62-6View Details
37415-62-6 -
 Mycophenolate Sodium CAS 37415-62-6View Details
Mycophenolate Sodium CAS 37415-62-6View Details
37415-62-6
