Montelukast
- CAS NO.:158966-92-8
- Empirical Formula: C35H36ClNO3S
- Molecular Weight: 586.18
- MDL number: MFCD05662278
- EINECS: 256-723-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
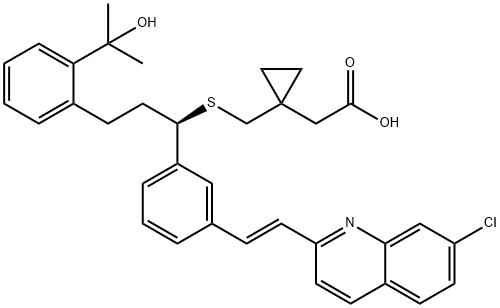
What is Montelukast?
Absorption
It has been observed that montelukast is quickly absorbed following administration by the oral route. The oral bioavailability documented for the drug is 64%. Furthermore, it seems that having a regular meal in the morning or even a high fat snack in the evening does not affect the absorption of montelukast.
Toxicity
The adverse effects associated with overdosage of montelukast include abdominal pain, somnolence, thirst, headache, vomiting, psychomotor hyperactivity, and less frequently, convulsion.
The oral LD50 value determined for mice and rats is >5000 mg/kg.
Montelukast has not been studied in pregnant women. Consequently, it should be used during pregnancy only if clearly needed.
Additionally, as it is unknown whether montelukast is excreted into human breast milk, there is also caution regarding the use of the medication in nursing mothers.
The plasma half-life of montelukast is somewhat prolonged in elderly patients, although no dosage adjustment is generally necessary.
Description
Montelukast (trade name Singulair) is a drug developed by Merck for maintenance treatment of asthma and relieving allergy symptoms. It is a leukotriene receptor antagonist that blocks leukotriene and its secondary ligands from cysteinyl leukotriene receptor 1 in the lungs and bronchial tubes.
Recently, a team led by Ludwig Aigner at Paracelsus Private Medical University of Salzburg (Austria) discovered an entirely new use for montelukast.
Noting that the signaling protein related to asthma-type inflammation is associated with age-related brain inflammation and impaired cognition in rodents, they gave the drug to “old” lab rats to see if their brains could function as well as their younger counterparts.
The researchers found that the?20-month-old rats did as well as the 4-month-olds in learning and memory tests. The older rats had less brain inflammation and increased neuron growth compared with untreated controls. This discovery could lead to new drugs for treating neurodegenerative diseases.
Originator
Singulair,Merck Pharmaceutical,Canada
The Uses of Montelukast
cardiostimulant,
Indications
Montelukast is indicated for:
(a) the prophylaxis and chronic treatment of asthma in adults and pediatric patients who are 12 months of age and older, although other regional health authorities specifically note this indication for adults and adolescents who are 15 years and older and also include indications for preventing day and night-time symptoms, and the treatment of acetylsalicylic acid-sensitive asthma;
(b) the prevention of exercise-induced bronchoconstriction (EIB) in patients who are 6 years of age and older, although other regional health authorities specifically note this indication for adults and adolescents who are 15 years and older; and
(c) the relief of symptoms of seasonal allergic rhinitis in patients 2 years of age and older and perennial allergic rhinitis in patients 6 months of age and older, although other regional health authorities specifically note the relief of seasonal allergic rhinitis symptoms for adults and adolescents who are 15 years and older.
Furthermore, some formulations like chewable montelukast tablets may also be specifically indicated by particular regulatory bodies for the prophylaxis and chronic treatment of asthma, including the prevention of day and night-time symptoms, the treatment of acetylsalicylic acid based asthma, and the prevention of exercise-induced bronchoconstriction in adult and pediatric patients aged 2 and older, between the ages 2 and 5, or between the ages of 6 and 14 years.
Moreover, when employed for such indications montelukast is considered effective as monotherapy or when combined with other medications indicated for the maintenance treatment of chronic asthma. For instance, montelukast and inhaled corticosteroids can be used concomitantly to demonstrate additive effects to control asthma or to decrease the necessary inhaled corticosteroid dose while still maintaining clinical stability.
Additionally, in patients who continue to experience asthma symptoms, montelukast can also be combined with an 'as required' short-acting beta-agonist, an inhaled corticosteroid, or inhaled corticosteroid paired with a long-acting beta-agonist.
Background
Montelukast was first approved for clinical use by the US FDA in 1998 as Merck's brand name Singulair. The medication is a member of the leukotriene receptor antagonist (LTRA) category of drugs. Although capable of demonstrating effectiveness, the use of such LTRAs like montelukast is typically in addition to or complementary with the use of inhaled corticosteroids or other agents in asthma step therapy. Regardless, in 2008-2009, there were FDA-led investigations into the possibility of montelukast to elicit neuropsychiatric effects like agitation, hallucinations, suicidal behaviour, and others in individuals who used the medication. And although these kinds of effects are currently included in the official prescribing information for montelukast, the drug still sees extensive use worldwide via millions of prescriptions annually and has since become available as a generic and as a brand name product.
Definition
ChEBI: Montelukast is a member of quinolines, a monocarboxylic acid and an aliphatic sulfide. It has a role as a leukotriene antagonist, an anti-asthmatic drug and an anti-arrhythmia drug. It is a conjugate acid of a montelukast(1-).
Manufacturing Process
Crotonaldehyde (3.23 mol) in 100 mL of 2-butanol was added dropwise to a
refluxing solution of 4-chloroaniline (3.23 mol), p-chloranil (3.23 mol) and HCl
conc. (808 mL) in 5.4 L of 2-butanol. After 2 hours of heating 2.7 L of solvent
was removed under vacuum at 60°C. Then 2 L of toluene was added to the
reaction mixture followed by removal of 2.5-3 L of solvent until a very pasty
solid formed. THF (2 L) was added and the mixture heated 30 min after which
it was cooled to 0°C. The solid was collected and washed with THF until pure
by tlc. The solid was then dissolved in aq. K2CO3/EtOAc and the organic phase
separated. The aqueous phase was extracted with EtOAc and the organic
phases combined, dried over MgSO4 and the solvent removed. The product
was crystallized in the minimum amount of EtOAc to give 328.08 g (57%) of
4-chloro-2-methylquinolin.
4-Chloro-2-methylquinalin was converted into 3-(2-(7-chloro)-2-
quinolinyl)ethenyl)benzaldehyde. Reaction was carried out according to a
method described in U.S. Pat. No. 4,851,409
To a degassed suspension of 3-(2-(7-chloro-2-quinolinyl)ethenyl)benzaldehyde
(0.34 mol) in toluene (700 mL) at 0°C was added 1.0 M vinylmagnesium
bromide in toluene/THF (370 mL). After stirring for 1 hour at 0°C, the
reaction was quenched by the addition of saturated NH4Cl solution (150 ml),
followed by H2O (500 mL) and HOAc (50 mL). The product was extracted with
EtOAc and the two-phase system was filtered through celite to remove an
insoluble precipitate. The aqueous phase was then re-extracted with EtOAc
(100 mL) and the combined organic layer was washed with H2O, followed by
brine. The solution was dried (MgSO4), and evaporated to give a dark yellow
residue which was purified by flash chromatography (EtOAc:hexane 1:5, then
1:3). The product was filtered from the column fractions to give a solid of 1-
(3-(2-(7-chloro-2-quinolinyl)ethenyl)phenyl)-2-propen-1-ol (melting point =
110-112°C). The filtrate was concentrated and the resulting residue was
recrystallized from EtOAc/hexane 1:4 to give a second crop of 15.1 g.
A degassed suspension of 1-(3-(2-(7-chloro-2-quinolinyl)ethenyl)phenyl)-2-propen-1-ol (46.6 mmol), n-Bu4NCl (93 mmol), LiOAcH2O (115 mmol), LiCl
(93 mmol), Pd(OAc)2 (1.4 mmol) and methyl 2-(2-iodophenyl)propanoate in
DMF (90 mL) was stirred for 2 hours at 100°C. The dark red solution was then
cooled to 0°C and poured into saturated NaHCO3 solution (500 mL). The
product was extracted with EtOAc and the organic layer was washed with H2O
followed by brine. The solvent was removed under vacuum and the residue
was purified by flash chromatography (EtOAc:hexane 1:10, 1:5 and 3:10) to
give a pale yellow foam of ethyl 2-(3(S)-(3-(2-(7-chloro-2-quinolinyl)ethenyl)
phenyl)-3-hydroxy-propyl)benzoate (18.9 g).
A mixture of anhydrous CeCl3 (164 mmol) in THF (500 mL) was refluxed
overnight using a Dean Stark trap filled with activated molecular sieves.
Methyl magnesium chloride (3.0 Molar solution in THF, 790 mmol) was added
dropwise over 30 min to the CeCl3 slurry at 0°C. After stirring 2 hours, the
mixture was cooled to -5°C and a toluene (600 mL) solution of the ethyl 2-
(3(S)-(3-(2-(7-chloro-2-quinolinyl)ethenyl)phenyl)-3-hydroxy-propyl)benzoate
(152 mmol) was added dropwise over 1 hour. The reaction mixture was stirred
another hour before the addition of 2 M HOAc (600 mL) and toluene (600
mL). The organic layer was washed with saturated aq. NaHCO3 and with brine.
Concentration in vacuo and purification of the residue by flash
chromatography (30% EtOAc in toluene) gave 63.48 g (91%) of the 2-(2-
(3(S)-(3-(2-(7-chloro-2-quinolinyl)ethenyl)phenyl)-3-hydroxypropyl)phenyl)-2-
propanol.
To a solution of BH3THF complex (1 M in THF, 262 mL) was added diethyl 1,1-
cyclopropanedicarboxylate (134 mmol) at 25°C under N2. The solution was
heated at reflux for 6 hours, cooled to r.t., and MeOH (300 mL) was cautiously
added. The solution was stirred for 1 hour and then concentrated to an oil.
The crude 2-(2-(3(S)-(3-(2-(7-chloro-2-quinolinyl)ethenyl)phenyl)-3-
hydroxypropyl)phenyl)-2-propanol was dissolved in CH2Cl2 (234 mL) and
SOCl2 (15.9 g, 134 mmol) was added dropwise over a period of 15 min at
25°C. After stirring for another 15 min, the mixture was washed with aqueous
NaHCO3. The organic extract was dried over Na2SO4, filtered and concentrated
to give quantitatively the 1,1-cyclopropanedimethanol cyclic sulfite.
To a solution of the 1,1-cyclopropanedimethanol cyclic sulfite (99 mmol) in
DMF (83 mL) was added NaCN (199 mmol). The mixture was heated to 90°C
for 20 hours. Upon cooling, EtOAc (400 mL) was added and the solution was
washed with saturated NaHCO3 solution (55 mL), H2O (4 times 55 mL),
saturated NaCl solution and dried over Na2SO4. The solution was concentrated
to give 7.1 g (65%) of 1-(hydroxymethyl)cyclopropaneacetonitrile.
To a solution of 1-(hydroxymethyl)cyclopropaneacetonitrile (42 g, 378 mmol)
in dry CH2Cl2 (450 mL) at -30°C was added Et3N (741 mmol) followed by
CH3SO2Cl (562 mmol) dropwise. The mixture was warmed to 25°C, washed
with NaHCO3, dried over Na2SO4 and concentrated in vacuo to give the
corresponding mesylate. The mesylate was then dissolved in DMF (450 mL)
and cooled to 0°C. Potassium thioacetate (55.4 g, 485 mmol) was added, and
the mixture was stirred at 25°C for 18 hours. EtOAc (1.5 L) was added, the
solution was washed with NaHCO3, dried over Na2SO4 and concentrated in
vacuo to give 45 g (70%) of 1-(acetythiomethyl)cyclopropaneacetonitrile.
To a solution of the 1-(acetythiomethyl)cyclopropaneacetonitrile (266 mmol)
in MeOH (1.36 L) was added H2O (84 mL) and conc. H2SO4(168 mL). The
mixture was heated to reflux for 20 hours, cooled to 25°C, H2O (1 L) was
added and the product was extracted with CH2Cl2. The organic extract was
washed with H2O and dried over Na2SO4. Concentration of the organic solution
gave 36 g (93%) of the methyl 1-(thiomethyl)cyclopropaneacetate.
To a solution of 2-(2-(3(S)-(3-(2-(7-chloro-2-quinolinyl)ethenyl)phenyl)-3-
hydroxypropyl)phenyl)-2-propanol in THF was dissolved in THF (1 mL) and
DMF (1 mL) at -40°C was added diisopropylethylamine (2.2 mmol) and then
methanesulfonyl chloride (2.2 mmol). The mixture was stirred 2 hours with
slow warming to -30°C. The methyl 1-(thiomethyl)cyclopropaneacetate (2.3
mmol) was added to the cloudy reaction mixture followed by dropwise
addition of potassium tert-butoxide/THF solution (4.4 mmol). The reaction
mixture was stirred at -30°C for 3.5 hours before quenching it with 25% aq
NH4OAc. Extraction with EtOAc, washing the organic layer with brine and
evaporation of the solvents left a residue that was purified by flash
chromatography (5%-10% EtOAc in toluene) giving 658 mg (53%) of methyl
1-((((R)-(3-(2-(7-chloro-2-quinolinyl)ethenyl)phenyl)-3-(2-(2-hydroxy-2-
propyl)phenyl)propyl)thio)methyl)cyclopropaneacetate.
Following the hydrolysis the methyl 1-((((R)-(3-(2-(7-chloro-2-quinolinyl)
ethenyl)phenyl)-3-(2-(2-hydroxy-2-propyl)phenyl)propyl)thio)methyl)
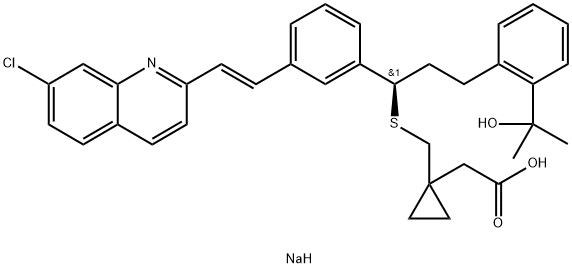
cyclopropaneacetate with NaOH was obtained the free acid: 4-((1(R)-(3-(2-(7-
chloro-2-quinolinyl)ethenyl)phenyl)-3-(2-(2-hydroxy-2-propyl)-phenyl)propyl)
thio)methyl)cyclopropaneacetic acid or sodium 1-(((1(R)-(3-(2-(7-chloro-2-
quinolinyl)ethenyl)phenyl)-3-(2-(2-hydroxy-2-propyl)phenyl)propyl)thio)
methyl) cyclopropaneacetate.
brand name
Singulair (Merck).
Therapeutic Function
Anti-asthmatic
Mechanism of action
Montelukast was developed from other weakly antagonistic quinoline derivatives. A number of changes can be made to the structure without the loss of activity. These include changing the double bond between the two aromatic rings to an ether linkage, reducing the quinoline ring, changing the chlorine to a fluorine, and/or exchanging the sulfur for an amide group.
Pharmacokinetics
Montelukast is a leukotriene receptor antagonist that demonstrates a marked affinity and selectivity to the cysteinyl leukotriene receptor type-1 in preference to many other crucial airway receptors like the prostanoid, cholinergic, or beta-adrenergic receptors. As a consequence, the agent can elicit substantial blockage of LTD4 leukotriene-mediated bronchoconstriction with doses as low as 5 mg. Moreover, a placebo-controlled, crossover study (n=12) demonstrated that montelukast is capable of inhibiting early and late phase bronchoconstriction caused by antigen challenge by 75% and 57% respectively.
In particular, it has been documented that montelukast can cause bronchodilation as soon as within 2 hours of oral administration. This action can also be additive to the bronchodilation caused by the concomitant use of a beta agonist. Nevertheless, clinical investigations performed with adults 15 years of age and older revealed that no additional clinical benefit is obtained when doses of montelukast greater than 10 mg a day are used.
Additionally, in clinical trials with adults and pediatric asthmatic patients aged 6 to 14 years, it was also determined that montelukast can reduce mean peripheral blood eosinophils by about 13% to 15% from baseline in comparison to placebo during double-blind treatment periods. At the same time, in patients aged 15 years and older who were experiencing seasonal allergic rhinitis, the use of montelukast caused a median reduction of 13% in peripheral blood eosinophil counts when compared to placebo as well.
Pharmacokinetics
Montelukast is a high-affinity, selective antagonist of the cysLT1 receptor. It is rapidly absorbed orally, with a bioavailability of 64%. Montelukast is 99% bound to plasma proteins and is extensively metabolized in the liver by CYP3A4 and CYP2C9 to oxidated products. CYP3A4 oxidizes the sulfur and the C-21 benzylic carbon, whereas CYP2C9 is selectively responsible for the methyl hydroxylation.
Clinical Use
Leukotriene receptor antagonist:
Prophylaxis of asthma
Seasonal allergic rhinitis
Side Effects
Montelukast did not demonstrate any significant adverse effects greater than placebo in clinical trials; however, because it is metabolized by the cytochrome P450 (CYP450) enzymes, its plasma levels should be monitored when coadministered with CYP450-inducing drugs, such as phenobarbital, rifampin, and phenytoin.
Metabolism
It has been determined that montelukast is highly metabolized and typically so by the cytochrome P450 3A4, 2C8, and 2C9 isoenzymes. In particular, it seems that the CYP2C8 enzymes play a significant role in the metabolism of the drug. Nevertheless, at therapeutic doses, the plasma concentrations of montelukast metabolites are undetectable at steady state in adults and pediatric patients.
Metabolism
Extensively metabolised in the liver by cytochrome P450 isoenzymes CYP3A4, CYP2A6, and CYP2C9. Excreted principally in the faeces via the bile. Metabolites have minimal therapeutic activity.
Properties of Montelukast
| Melting point: | 145-148 °C(Solv: toluene (108-88-3); methanol (67-56-1)) |
| Boiling point: | 750.5±60.0 °C(Predicted) |
| Density | 1.272±0.06 g/cm3(Predicted) |
| storage temp. | Store at 2-8°C |
| solubility | DMF: 2 mg/ml,DMSO: 2 mg/ml,Ethanol: insol,PBS (pH 7.2): insol |
| pka | 4.76±0.10(Predicted) |
| form | A solid |
| color | Light yellow to yellow |
| CAS DataBase Reference | 158966-92-8(CAS DataBase Reference) |
| EPA Substance Registry System | Cyclopropaneacetic acid, 1-[[[(1R)-1-[3-[(1E)-2-(7-chloro-2-quinolinyl)ethenyl]phenyl]-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl]thio]methyl]- (158966-92-8) |
Safety information for Montelukast
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P362:Take off contaminated clothing and wash before reuse. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Montelukast
Montelukast manufacturer
Allmpus Laboratories Pvt Ltd
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran
![Methyl [E]-2-[3-(S)-[3-[2-(7-Chloro-2-quinolinyl)ethenyl]phenyl]-3-hydroxypropyl]benzoate](https://img.chemicalbook.in/CAS/GIF/142569-69-5.gif)


You may like
-
 158966-92-8 Montelukast Sodium- IP/BP 98%View Details
158966-92-8 Montelukast Sodium- IP/BP 98%View Details
158966-92-8 -
 Montelukast 99%View Details
Montelukast 99%View Details -
 158966-92-8 Montelukast 98%View Details
158966-92-8 Montelukast 98%View Details
158966-92-8 -
 Montelukast 158966-92-8 98%View Details
Montelukast 158966-92-8 98%View Details
158966-92-8 -
 Montelukast 99%View Details
Montelukast 99%View Details -
 Montelukast 98%View Details
Montelukast 98%View Details -
 Montelukast 158966-92-8 98%View Details
Montelukast 158966-92-8 98%View Details
158966-92-8 -
 158966-92-8 98.17View Details
158966-92-8 98.17View Details
158966-92-8
