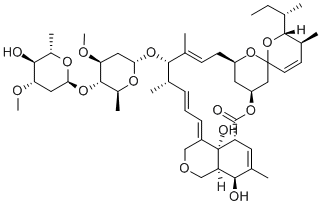
Monensin sodium salt
Synonym(s):Monensin A sodium salt;Monensin, Sodium Salt, High Purity - CAS 22373-78-0 - Calbiochem
- CAS NO.:22373-78-0
- Empirical Formula: C36H63NaO12
- Molecular Weight: 710.87
- MDL number: MFCD00077826
- EINECS: 244-941-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18
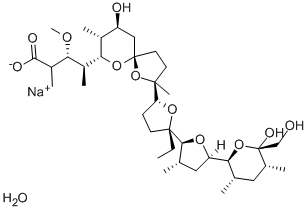
What is Monensin sodium salt?
Description
Monensin is a naturally occuring ionophorous antibiotic that preferentially forms complexes with monovalent cations to enable transport across lipid membranes. Through its ability to affect pH and the sodium-potassium balance of a cell, monensin can induce cell death in Gram-positive bacteria such as Micrococcus, Bacillus, and Staphylococcus (MICs = 1-12.5 μg/ml), reduce proliferation of P. falciparum and Coccicdium protozoa, and also prevent replication of certain viruses.
Chemical properties
LIGHT CREAM AMORPHOUS POWDER
The Uses of Monensin sodium salt
Monensin sodium salt is used in potentiometric and spectroscopic studies of alkali metal ion complexes. Monensin (Na) is a polyether small molecule ionophore, capable of forming stable complexes to monovalent cations with specific affinity for Na+. Monensin presents a highly lipophilic scaffold around the bound ion, allowing movement of the complex through the lipid bilayer and consequent transport of the ion out of the cell. Excessive flux of ions out of the cell produces a cytotoxic effect and generates the antibiotic properties of Monensin.
The Uses of Monensin sodium salt
Poliether antibiotic. Coccidiostat.
The Uses of Monensin sodium salt
antifungal
The Uses of Monensin sodium salt
antibacterial
What are the applications of Application
Monensin Sodium Salt is a monovalent cation ionophore and transporter
General Description
Monensin is a polyether ionophoric antibiotic, which is produced by Streptomyces cinnamonensis. It is used to treat bacterial, fungal and parasitic infections. Monensin prevents the growth of colon cancer cells. It facilitates the transport of sodium and potassium ions between intracellular and extracellular spaces. Monensin prevents coccidiosis?in poultry production.
Biochem/physiol Actions
Na+ ionophore; blocks glycoprotein secretion; may induce catecholamine secretion from chromaffin cells. Useful in potentiometric and spectroscopic studies of alkali metal ion complexes.
Storage
-20°C
Purification Methods
Crystallise it from EtOH/H2O [Cox et al. J Am Chem Soc 107 4297 1985].
References
Aowicki and Huczynski (2013), Structure and antimicrobial properties of monensin A and its derivatives: summary of the achievements; Res. Int. 2013 742149 Kallen et al. (1993), Monensin inhibits synthesis of plasma membrane sphingomyelin by blocking transport of ceramide through the Golgi: evidence for two sites of sphingomyelin synthesis in BHK cells; Biophys. Acta 1166 305 Boss et al. (1984), Monensin-induced swelling of Golgi apparatus cisternae mediated by a proton gradient; J. Cell. Biol. 34 1 Mollenhauer et al. (1990), Alteration of intracellular traffic by monensin; mechanism, specificity and relationship to toxicity; Biophys. Acta 1031 225 Hafner et al. (2021), The Cardenolide Glycoside Acovenoside A Interferes with Epidermal Growth Factor Receptor Trafficking in Non-Small Cell Lung Cancer Cells; Pharmacol. 12 611657 Oh-Hashi et al. (2021), Comparative Analysis of CREB3 and CREB3L2 Protein Expression in HEK293 Cells; J. Mol. Sci. 22 2767
Properties of Monensin sodium salt
| Melting point: | 267-269 C |
| alpha | 57.3 º (c=4.0, in MeOH) |
| storage temp. | 2-8°C |
| solubility | Soluble in methanol at 50mg/ml |
| form | Off-white to beige solid |
| color | White |
| BRN | 4122200 |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 1 month. |
| EPA Substance Registry System | Monensin, monosodium salt (22373-78-0) |
Safety information for Monensin sodium salt
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H300:Acute toxicity,oral H319:Serious eye damage/eye irritation H334:Sensitisation, respiratory H373:Specific target organ toxicity, repeated exposure H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Monensin sodium salt
| InChIKey | XOIQMTLWECTKJL-BEMBKCOJSA-M |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Monensin sodium CAS 22373-78-0View Details
Monensin sodium CAS 22373-78-0View Details
22373-78-0 -
 Monensin Sodium Salt (MSN) CAS 22373-78-0View Details
Monensin Sodium Salt (MSN) CAS 22373-78-0View Details
22373-78-0 -
 Monensin sodium salt 90% (HPLC) CAS 22373-78-0View Details
Monensin sodium salt 90% (HPLC) CAS 22373-78-0View Details
22373-78-0 -
 Monensin Sodium CAS 22373-78-0View Details
Monensin Sodium CAS 22373-78-0View Details
22373-78-0 -
 Monensin sodium salt hydrate CAS 22373-78-0View Details
Monensin sodium salt hydrate CAS 22373-78-0View Details
22373-78-0 -
 Monensin sodium CAS 22373-78-0View Details
Monensin sodium CAS 22373-78-0View Details
22373-78-0 -
 Monensin sodium salt CAS 22373-78-0View Details
Monensin sodium salt CAS 22373-78-0View Details
22373-78-0 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0
