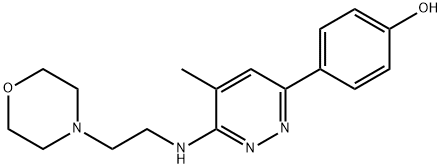
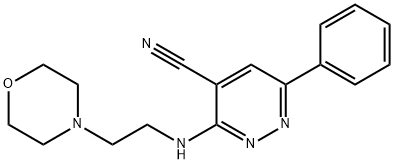
MINAPRINE DIHYDROCHLORIDE
Synonym(s):N-(4-Methyl-6-phenyl-3-pyridazinyl)-4-morpholineethanamine
- CAS NO.:25905-77-5
- Empirical Formula: C17H22N4O
- Molecular Weight: 298.38
- MDL number: MFCD00083410
- EINECS: 247-329-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
What is MINAPRINE DIHYDROCHLORIDE?
Originator
Cantor,Clin Midy,France,1979
The Uses of MINAPRINE DIHYDROCHLORIDE
muscle relaxant (skeletal)
The Uses of MINAPRINE DIHYDROCHLORIDE
Minaprine is a central nervous system (CNS) drug through the use of analog synthesis and CYP2D6 enzyme kinetic analyses.
Background
Minaprine is a psychotropic drug which has proved to be effective in the treatment of various depressive states. Like most antidepressants minaprine antagonizes behavioral despair. Minaprine is an amino-phenylpyridazine antidepressant reported to be relatively free of cardiotoxicity, drowsiness, and weight gain.
Indications
For the treatment of depression
Definition
ChEBI: Minaprine is a member of pyridazines, a secondary amine and a member of morpholines. It has a role as an antidepressant, a serotonin uptake inhibitor, a dopamine uptake inhibitor, a cholinergic drug and an antiparkinson drug.
Manufacturing Process
(a) Preparation of the free base: A mixture comprising 0.1 mol (20.4 g) of 3-chloro-4-methyl-6-phenylpyridazine and 0.2 mol (26.2 g) of N-(2-aminoethyl)-morpholine in 100 ml of n-butanol, with a pinch of copper powder, was heatedunder reflux for 12 hours. At the end of this time, the hot solution was pouredinto 200 ml of cold water. The resulting mixture was filtered through asintered glass filter and the precipitate washed with ether. The filtrate and theether washings were placed in a separating funnel and extracted with two 150ml portions of ether. The ethereal layer was then extracted with about 250 mlof N sulfuric acid.
The acid solution was made alkaline with a 10% aqueous solution of sodiumcarbonate, and left to crystallize overnight.
The solution was filtered, yielding the colorless needles which wererecrystallized from isopropanol. The yield was 15 g (53%).
(b) Preparation of the hydrochloride: The base was dissolved in the smallestamount possible of anhydrous acetone. Double that volume of anhydrousether was added, and a stream of hydrogen chloride gas was passed throughthe solution. The hydrochloride salt obtained was recrystallized from absolutealcohol. The yield after recrystallization was 17 g (90%).
brand name
Cantor (Sanofi).
Therapeutic Function
Antidepressant
Pharmacokinetics
Minaprine is an amino-phenylpyridazine antidepressant reported to be relatively free of cardiotoxicity, drowsiness, and weight gain. Similar to other antidepressant treatments, minaprine attenuates the beta-adrenergic receptor function. Studies have also shown that minaprine improves memory consolidation and that repeated drug administration leads to potentiation of this effect. Moreover, the effects of minaprine on memory consolidation are related to its dopaminergic action.
Metabolism
Hepatic. Cytochrome P4502D is responsible for the 4-hydroxylation of minaprine to 4-hydroxyminaprine.
Properties of MINAPRINE DIHYDROCHLORIDE
| Melting point: | 122° |
| Boiling point: | 531.2±50.0 °C(Predicted) |
| Density | 1.156±0.06 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | DMSO : ≥ 35 mg/mL (117.30 mM) |
| pka | 6.80±0.10(Predicted) |
| form | neat |
Safety information for MINAPRINE DIHYDROCHLORIDE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for MINAPRINE DIHYDROCHLORIDE
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran
You may like
-
![Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
61397-56-6 -
 287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 -
![2033-24-1 2,2-Dimethyl-1,3-Dioxane-4,6-Dione [Meldrum Acid] 98%](https://img.chemicalbook.in//Content/image/CP5.jpg) 2033-24-1 2,2-Dimethyl-1,3-Dioxane-4,6-Dione [Meldrum Acid] 98%View Details
2033-24-1 2,2-Dimethyl-1,3-Dioxane-4,6-Dione [Meldrum Acid] 98%View Details
2033-24-1 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 CIS- BROMO BENZOATEView Details
CIS- BROMO BENZOATEView Details
61397-56-6 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
![1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
221615-75-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3