MEVINPHOS
Synonym(s):(E)-3-(Dimethoxyphosphinyloxy)-2-butenoic acid methyl ester;cis-Mevinphos solution
- CAS NO.:26718-65-0
- Empirical Formula: C7H13O6P
- Molecular Weight: 224.15
- MDL number: MFCD00048060
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 17:34:36
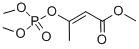
What is MEVINPHOS?
The Uses of MEVINPHOS
Mevinphos is used to control chewing and sucking insects in a wide range of crops.
Definition
ChEBI: Mevinphos is a dialkyl phosphate and an organophosphate insecticide. It has a role as an EC 3.1.1.7 (acetylcholinesterase) inhibitor, an acaricide, an agrochemical and an avicide. It is functionally related to a methyl 3-hydroxybut-2-enoate.
Metabolic pathway
Technical mevinphos consists of two stereochemical isomers in the E:Z ratio of about 7:3. Mevinphos is characterised by a high rate of metabolic breakdown, such that crops can be harvested within a few hours of its application. The compound is also rapidly translocated within plants. There are considerable differences in the metabolism of the two isomers. In general, the E-isomer (the more toxic, more active acetylcholinesterase inhibitor and more effective insecticide) is detoxified more quickly in plants but more slowly in animals. The major route of detoxification in plants is through hydrolysis to give dimethyl phosphate and methyl acetoacetate with hydrolysis of the carboxylic ester function being of lesser importance. Demethylation of the more toxic Eisomer via a glutathione-based transalkylation reaction is an important route in mammals, whereas the 2-isomer is hydrolysed to dimethyl phosphate .
Degradation
Mevinphos is hydrolysed in alkaline solutions (PM). The DT50s for the technical material at pH 6, 7 and 10 were 94 days, 30 days and 8 hours respectively (Porter et al., 1964). The E-isomer (1) is hydrolysed more quickly than the Z-isomer (2). Under alkaline conditions, the products of hydrolysis of the E-isomer were shown to be dimethyl phosphate (3), monomethyl phosphate (4), acetone (S), the E-mevinphos carboxylic acid (6) and the desmethyl E-mevinphos carboxylic acid (7). A small amount of desmethyl E-mevinphos (8) was also detected. The Z-isomer gave very little of the desmethyl Z-mevinphos carboxylic acid (10) indicating that the attack of hydroxyl ion on the 2-isomer was almost entirely on phosphorus, whereas there was additional nucleophilic attack on the carboxylic ester function in the case of the E-isomer which was shown to gwe dimethyl phosphate (3) and acetone (5). Acid hydrolysis resulted in demethylation of both isomers with the desmethyl E-mevinphos (8) and desmethyl Z-mevinphos (9) being detected in addition to dimethyl phosphate (3) and monomethyl phosphate (4) (Spencer et al., 1958) (see Scheme 1).
Properties of MEVINPHOS
| vapor pressure | 1.7×10-2 Pa (20 °C) |
| storage temp. | 0-6°C |
| Water Solubility | Totally miscible |
Safety information for MEVINPHOS
Computed Descriptors for MEVINPHOS
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

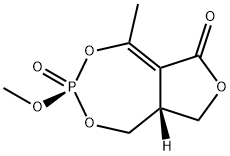


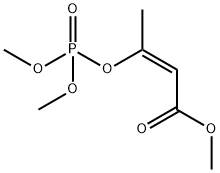
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1