METHYLPHOSPHONIC DICHLORIDE
Synonym(s):Dichloromethylphosphine oxide;Methanephosphonyl chloride;Methylphosphonodichloridic acid;Methylphosphonyl chloride;Methylphosphonyl dichloride
- CAS NO.:676-97-1
- Empirical Formula: CH3Cl2OP
- Molecular Weight: 132.91
- MDL number: MFCD00002071
- EINECS: 211-634-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
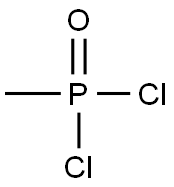
What is METHYLPHOSPHONIC DICHLORIDE?
Description
Methyl phosphonic dichloride is a low meltingsolid or colorless liquid. Molecular weight = 132.91;Boiling point = 162℃; Freezing/Melting point 5 32℃;Flash point = $ 50℃ (oc). Mixes violently with water.Potential Exposure: Used as a chemical intermediate inpesticide manufacture
Chemical properties
Methyl phosphonic dichloride is a low melting solid or colorless to pale yellow liquid. Pungent odor.
The Uses of METHYLPHOSPHONIC DICHLORIDE
suzuki reaction
Production Methods
The oxidation of lowvalency organophosphorus compounds is important mainly in the case of the thiophosphonic acid chlorides. This method is used in the production of methylphosphonic acid dichloride by reaction of methyldichlorophosphine with sulfuryl chloride or chlorosulfuric acid. Elemental sulfur reacts with MePCl2 in the presence of catalytic quantities of tetraalkylphosphonium salts to form methylthiophosphonic acid dichloride.
General Description
Strongly irritates skin. Contact may destroy or irreversibly alter skin tissue. Very toxic by ingestion, inhalation, or by skin absorption. Combustible, though may be difficult to ignite.
Air & Water Reactions
Fumes in moist air to form hydrogen chloride. Reacts with water to form hydrochloric acid, reaction may be violent.
Reactivity Profile
METHYLPHOSPHONIC DICHLORIDE is incompatible with water, strong oxidizing agents, alcohols, bases (including amines).. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].
Health Hazard
Poisonous if inhaled or swallowed. Contact causes severe burns to skin and eyes.
Fire Hazard
METHYLPHOSPHONIC DICHLORIDE may burn but does not ignite readily. May ignite other combustible materials (wood, paper, oil, etc.). Reacts violently with water. Flammable poisonous gases may accumulate in tanks and hopper cars. Runoff to sewer may create fire or explosion hazard. Contact causes severe burns to skin and eyes. Runoff from fire control or dilution water may cause pollution. Violent reaction with water.
Safety Profile
Poison by inhalation. A corrosive irritant to the eyes, skin, and mucous membranes. When heated to decomposition it emits toxic fumes of Cland POx.
Potential Exposure
Highly flammable; mists or Vapors may form explosive mixture with air. Reacts with moist air forming fumes of hydrogen chloride; may spontaneously ignite. Reacts with water or alcohol, forming hydrochloric acid. The reaction may be violent and ignite unreacted material. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, amines, ethers. May react violently, possibly explosively, when mixed with ethers and trace amounts of metal salts.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, getmedical attention. If victim is conscious, administer wateror milk. Do not induce vomiting. Keep victim quiet andmaintain normal body temperature. Medical observation isrecommended for 24- 48 h after breathing overexposure, aspulmonary edema may be delayed. As first aid for pulmo-nary edema, a doctor or authorized paramedic may consideradministering a corticosteroid spray.
storage
Color Code—White: Corrosive or Contact Hazard;Store separately in a corrosion-resistant location. Prior toworking with this chemical you should be trained on itsproper handling and storage. Store in tightly closed containers in a cool, well-ventilated area. Sources of ignition, suchas smoking and open flames, are prohibited where thischemical is handled, used, or stored. Use only nonsparkingtools and equipment, especially when opening and closingcontainers of this chemical. Wherever this chemical is used,handled, manufactured, or stored, use explosion-proof electrical equipment and fittings
Shipping
UN9206 Methyl phosphonic dichloride, Hazard class: 6.1; Labels: 6.1-Poisonous materials, 8-Corrosive material. Domestic (United States), Inhalation Hazard Zone B. UN3390 Toxic by inhalation liquid, corrosive, n.o.s. with an LC50 # 1000 mL/m3 and saturated vapor concentration ≥ 10 LC50 Hazard Class: 6.1; Labels: 6.1- Poisonous materials, 8-Corrosive material, Technical Name Required, Inhalation Hazard Zone B
Purification Methods
Methylphosphonic dichloride [676-97-1] M 132.9, m 33o, 33-37o, b 53-54o/10mm, 64-6 7o/20.5mm, 86o/44mm, 162o/760mm, d 4 1.4382. Fractionally redistil it until the purity as checked by hydrolysis and acidimetry for Clis correct and the distillate should solidify on cooling. [Kinnear & Perren J Chem Soc 3437 1952, Crofts & Kosolapoff J Am Chem Soc 75 3379 1952, for IR see McIvor et al. Can J Chem 34 1611 1956, Beilstein 4 IV 3509.]
Incompatibilities
Reacts violently with water, alcohols,forming hydrochloric acid/hydrogen chloride vapor. Thereaction may be violent. Corrodes metals. Incompatiblewith strong oxidizers, alcohols, bases (including amines).May react violently, possibly explosively, when mixed withethers and trace amounts of metal salts.
Waste Disposal
Use a licensed professional waste disposal service to dispose of this material. Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed
Properties of METHYLPHOSPHONIC DICHLORIDE
| Melting point: | 31-34 °C |
| Boiling point: | 163 °C(lit.) |
| Density | 1.468 g/mL at 25 °C(lit.) |
| vapor pressure | 760 mm Hg ( 163 °C) |
| Flash point: | >110°C |
| solubility | Acetonitrile (Slightly), Chloroform |
| form | solid |
| Water Solubility | Soluble in water. |
| Sensitive | Moisture Sensitive |
| BRN | 1071305 |
| Stability: | Stable, but may decompose in the presence of water or moisture. Incompatible with water, strong bases, strong oxidizing agents. |
| CAS DataBase Reference | 676-97-1 |
| EPA Substance Registry System | Methylphosphonic dichloride (676-97-1) |
Safety information for METHYLPHOSPHONIC DICHLORIDE
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H330:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for METHYLPHOSPHONIC DICHLORIDE
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



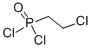

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1