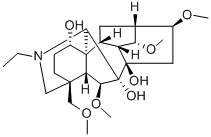
METHYLLYCACONITINE CITRATE
Synonym(s):[1α,4(S),6β,14α,16β]-20-Ethyl-1,6,14,16-tetramethoxy-4-[[[2-(3-methyl-2,5-dioxo-1-pyrrolidinyl)benzoyl]oxy]methyl]aconitane-7,8-diol citrate salt;MLA
- CAS NO.:112825-05-5
- Empirical Formula: C43H58N2O17
- Molecular Weight: 874.92
- MDL number: MFCD00153837
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
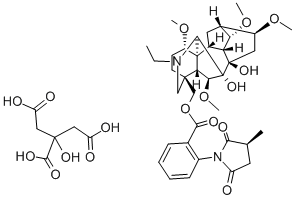
What is METHYLLYCACONITINE CITRATE?
The Uses of METHYLLYCACONITINE CITRATE
Methyllycaconitine citrate salt has been used as an α7 nicotinic acetylcholine receptor (α7 nAChR) antagonist:
- to study its effects on inflammatory response in rats post nicotine treatment
- to block the activity of galantamine
- to study its effects on the hepatic branch of the vagus nerve (hVNS) in rats
What are the applications of Application
Methyllycaconitine citrate is a potent antagonist for α7-containing neuronal nicotinic receptors
Biological Activity
methyllycaconitine citrate(mla) is an antagonist of α7-containing neuronal nicotinic acetylcholine receptors (nachrs; ki = 1.4 nm)[1,2].mla inhibits the decreased cell viability induced by aβ25-35, pretreatment with 5 and 10 μm. aβ25-35 treatment increases lc3-ii levels, which is inhibited by administration of methyllycaconitine citrate. mla also inhibits aβ-induced autophagosome accumulation in sh-sy5y cells[3].mla inhibits methamphetamine(meth)-induced climbing behavior by 50%. mla prevents a decrease in striatal synaptosome dopamine (da) uptake, mla significantly attenuates meth-induced neurotoxicity at 72 h post-treatment. mla fully prevents microglial activation at 24 h post-treatment and tending to confirm its neuroprotective activity[4].[1]. kalappa b i, sun f, johnson s r, et al. a positive allosteric modulator of α7 nachrs augments neuroprotective effects of endogenous nicotinic agonists in cerebral ischaemia. brit.j.pharmacol, 2013, 169(8): 1862-1878.[2]. ward j m, cockcroft v b, lunt g g, et al. methyllycaconitine: a selective probe for neuronal α-bungarotoxin binding sites. febs lett, 1990, 270(1-2): 45-48 .[3]. zheng x, et al. methyllycaconitine alleviates amyloid-β peptides-induced cytotoxicity in sh-sy5y cells. plos one, 2014, 9(10): e111536.[4]. escubedo e, et al. methyllycaconitine prevents methamphetamine-induced effects in mouse striatum: involvement of alpha7 nicotinic receptors. j pharmacol exp ther, 2005, 315(2): 658-67.
Biochem/physiol Actions
Methyllycaconitine (MLA) is an α7 nicotinic acetylcholine receptor (α7 nAChR) antagonist. It is a norditerpenoid alkaloid synthesized by several species of Delphinium. MLA binds to the binding site of neuronal α-bungarotoxin. Low doses of MLA are associated with improvement of cognition in animals.
Properties of METHYLLYCACONITINE CITRATE
| storage temp. | −20°C |
| solubility | H2O: 42 mg/mL |
| form | solid |
| color | white |
Safety information for METHYLLYCACONITINE CITRATE
Computed Descriptors for METHYLLYCACONITINE CITRATE
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Methyllycaconitine citrate salt CAS 112825-05-5View Details
Methyllycaconitine citrate salt CAS 112825-05-5View Details
112825-05-5 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
