Methylamine
Synonym(s):Monomethylamine
- CAS NO.:74-89-5
- Empirical Formula: CH5N
- Molecular Weight: 31.06
- MDL number: MFCD00008104
- EINECS: 200-820-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
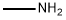
What is Methylamine?
Description
Methylamine is a colorless gas with a fish- or ammonia-like odor; at low concentrations a fishy odor. Shipped as a liquefied compressed gas. The odor threshold is 3.2 ppm. Molecular weight= 31.07; Specific gravity (H2O:1)= 0.7 (liquid);Boiling point=6℃; Freezing/ Melting point=94℃; Relative vapor density (air= 1)= 1.08; Vapor pressure= 3 atm; Flash point=flammable gas; Autoignition temperature=430℃. Explosive limits: LEL= 4.9%; UEL= 20.7%. Hazard Identification (based on NFPA-704 M Rating System): Health 3, Flammability 4, Reactivity 0. Soluble in water.
Chemical properties
colourless gas (or solution in water or methanol)
Chemical properties
Methylamine is a colorless, fi sh-like smelling gas at room temperature. It is used in a variety of industries, such as the manufacture of dyestuffs, treatment of cellulose, acetate rayon, as a fuel additive, rocket propellant, and in leather tanning processes.
Chemical properties
Methylamine is a colorless gas with a fish- or ammonia-like odor; at low concentrations a fishy odor. Shipped as a liquefied compressed gas. The odor threshold is 3.2 ppm.
Chemical properties
Methylamine is a derivative of ammonia in which a methyl group is substituted for a hydrogen (Schweizer et al 1978). Its reactivity is governed primarily by the unshared pair of electrons on the nitrogen, therefore methylamine is a strongly alkaline base whose most characteristic reaction is the formation of salts with acids. It will react with acid halides and acid anhydrides to form N-substituted amines. Methylamine reacts with nitrous acid to form methanol with liberation of nitrogen. It is capable of reacting with aldehydes to form aldimines or Schiffs bases (Astle 1961).
Physical properties
Colorless, flammable gas with a strong ammonia-like odor. An experimentally determined recognition odor threshold concentration of 21 ppbv was reported by Leonardos et al. (1969). Odor threshold concentrations of 4.7 ppmv and 35 ppbv were experimentally determined by Nishida et al. (1979) and Nagata and Takeuchi (1990), respectively.
The Uses of Methylamine
Methylamine is used in dyeing and tanning;in photographic developer, as a fuel additive,and as a rocket propellant. It is also usedin organic synthesis and as a polymerizationinhibitor. It occurs in certain plants, such asMentha aquatica.
The Uses of Methylamine
Intermediate for accelerators, dyes, pharmaceuticals, insecticides, fungicides, surface active agents, tanning, dyeing of acetate textiles, fuel additive, polymerization inhibitor, component of paint removers, solvent, photographic developer, rocket propellent.
The Uses of Methylamine
Tanning and dyeing industries; fuel additive; chemical intermediate in the production of pharmaceuticals, insecticides, and surfactants
Definition
ChEBI: The simplest of the methylamines, consisting of ammonia bearing a single methyl substituent.
Production Methods
Several methods are currently used for synthesis of methylamine. Virtually all produce a mixture of primary, secondary, and tertiary amines which can be continuously separated by distillation and extraction. The most commonly used synthesis involves heating ammonium chloride and methyl alcohol (ratio varies from 2:1 to 6:1, depending on desired ratio of amines) to about 300°C in the presence of a catalyst such as zinc chloride. Alternatively, methylamine can be synthesized by heating ammonium chloride and formaldehyde in the presence of H2 and a hydrogenation catalyst such as nickel or platinum. Methylamine is generally marketed as a liquid or a 33% aqueous solution (HSDB 1988).
Definition
A colorless flammable gas that smells like ammonia. It is the simplest primary amine, used for making herbicides and other organic chemicals.
Definition
methylamine: A colourless flammablegas, CH3NH2; m.p. –93.5°C; b.p.–6.3°C. It can be made by a catalyticreaction between methanol and ammoniaand is used in the manufactureof other organic chemicals.
General Description
A colorless gas or a liquid. Pungent fishy odor resembling odor of ammonia. The liquid boils at 20.3°F hence vaporizes rapidly when unconfined. Vapors are heavier than air and may collect in low-lying areas. Easily ignited under most conditions. Under prolonged exposure to intense heat the containers may rupture violently and rocket. Used for making pharmaceuticals, insecticides, paint removers, surfactants, rubber chemicals.
Air & Water Reactions
Highly flammable. Very soluble in water; the solutions are strongly basic and therefore corrosive. Liquid fumes in air.
Reactivity Profile
METHYLAMINE neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated in combination with strong reducing agents, such as hydrides.
Hazard
(Gas and liquid) Dangerous fire risk. Explosive limits in air 5–21%. Strong irritant to tissue. Eye, skin and upper respiratory tract irritant.
Health Hazard
VAPOR: Irritating to eyes, nose and throat. If inhaled will cause coughing or difficult breathing. LIQUID: Will burn skin and eyes.
Health Hazard
Exposures to methylamine are known to cause adverse health effects among occupational workers. The workers demonstrate symptoms of toxicity that include, but are not limited to, irritation to the eyes, nose, and throat. Studies have indicated that the compound causes injury to the eyes through corneal opacities and edema hemorrhages in the conjunctiva, and injury to the liver. Studies of Guest and Varma indicated no signifi cant deleterious effects on the internal organs or skeletal deformities in experimental mice
Health Hazard
Most of the studies on the toxicity of methylamine suggest it acts locally as an
irritant and a sensitizer. Vapors result in eye irritation with tearing and inflammation
with repeated exposure capable of inducing corneal damage. Exposure by
inhalation irritates the mucous membranes of the nose, throat and lung, leading to
coughing and respiratory distress. Skin contact with methylamine can lead to
burns and dermatitis (Beard and Noe 1981). First aid for skin exposure requires
immediate flushing with water.
Persons exposed to methylamine can detect a faint fishlike odor at less than 10
p.p.m. Little irritation occurs however, above 20 p.p.m. Methylamine can induce
temporary irritation of the eyes, nose, and throat. The permissible exposure level
(PEL) has been set at 10 p.p.m. (OSHA 1977) and the level immediately dangerous
to life or health (IDLH) is 100 p.p.m. (Standards Completion Program,
OSHA and NIOSH 1978).
Health Hazard
250 ppm produced damage to respiratorymucosa of the nasal turbinates. Exposureto 750 ppm produced severe body weightlosses, liver damage, and nasal degenerativechanges.
Any adverse health effects in humans dueto methylamine, other than its irritant action,is unknown.
LC50 value, inhalation (mice): 2400 mg/kg/2 h.
Fire Hazard
FLAMMABLE. POISONOUS GASES MAY BE PRODUCED IN FIRE. Containers may explode in fire. Flashback along vapor trail may occur. Vapor may explode if ignited in an enclosed area. Toxic nitrogen oxides may be formed. Vapors are heavier than air and may travel considerable distance to a source of ignition and flash back.
Industrial uses
Methylamine and its hydrochloride salt are widely used in organic synthesis for introducing the methylamino group. In 1976, industrial consumption was 32,000 tons/year. One of its most important uses is in the preparation of amide-type surfactants. It is also used in the preparation of drugs such as adrenaline and synthetic caffeine. It serves as the base for more than twenty commercial products, among those included are photographic developers, insecticides, and antihistamine drugs (Beard and Noe 1981). It is also widely used in tanning and has been used in the separation of aromatics from aliphatic hydrocarbons (Sittig 1981).
Safety Profile
Poison by subcutaneous route. Moderately toxic by inhalation. A severe skin irritant. Mutation data reported. A strong base. Flammable gas at ordinary temperature and pressure. Very dangerous fire hazard when exposed to heat, flame, or sparks. Explosive when exposed to heat or flame. To fight fire, stop flow of gas. Forms an explosive mixture with nitromethane. When heated to decomposition it emits toxic fumes of NOx. See also AMINES.
Potential Exposure
Methylamine is used in organic synthesis; a starting material for N-oleyltaurine, a surfactant; and p-N-methylaminophenol sulfate, a photographic developer. It has possible uses in solvent extraction systems in separation of aromatics from aliphatic hydrocarbons. It is also used in the synthesis of many different pharmaceuticals; pesticides and rubber chemicals.
First aid
If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least 15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart action has stopped. Transfer promptly to a medical facility. When this chemical has been swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit. Medical observation is Methylamine 1755 recommended for 2448 h after breathing overexposure, as pulmonary edema may be delayed. As first aid for pulmonary edema, a doctor or authorized paramedic may consider administering a corticosteroid spray.
Carcinogenicity
Methylamine was positive in the mouse lymphoma assay and negative in the Ames assay.
Source
Methylamine was detected in cauliflower (65 ppm), carrots (3,970 ppm), tea leaves (50 ppm), red and white cabbage (3.4 to 22.7 ppm), corn (27 ppm), kale leaves (16.6 ppm), barley seeds (4.5 ppm), epidermis of apples (4.5 ppm), celery (6.4 ppm), sweetflag, celandine, and tobacco leaves (Duke, 1992).
Environmental Fate
Photolytic. The rate constant for the reaction of methylamine and OH radicals in the atmosphere
at 300 K is 1.3 x 10-13 cm3/molecule?sec (Hendry and Kenley, 1979).
Low et al. (1991) reported that the photooxidation of aqueous primary amine solutions by UV
light in the presence of titanium dioxide resulted in the formation of ammonium and nitrate ions.
Chemical/Physical. In an aqueous solution, chloramine reacted with methylamine to form Nchloromethylamine
(Isaac and Morris, 1983).
Reacts with acids forming water-soluble salts.
Metabolism
Methylamine is a normal body constituent in several species and is known to be
generated endogenously from epinephrine (Schayer et al 1952) and creatine
(Davis and DeRopp 1961). It has also recently been detected in the urine of male
CBA/cA mice treated with N-methylformamide (Kestell et al 1985). Mammalian
metabolism of methylamine is rapid yet the enzymes involved are not yet known.
Simehnhoff (1975) suggested that methylamine is methylated to dimethylamine as
it appeared not to be oxidized by amine oxidases yet was rapidly absorbed and not
excreted in the urine. Dar et al (1985) conducted studies using methyl-[14C]-
labeled methylamine injected i.p. into rats to assess the role of monoamine oxidase
in the metabolism of methylamine in the rats. Methylamine underwent rapid
oxidation as more than 30% of the 14C was recovered as 14CO2 in the first 2-6 h
following exposure and 52% was expired in the first 24 h. Pretreatment of the rats
with long acting monoamine oxidase inhibitors significantly inhibited methylamine
metabolism, however short term inhibitors were without effect. Combinations
of the drugs suggested that monoamine oxidase was not responsible for
metabolism of methylamine and that a closely related enzyme such as methylamine
oxidase, previously proposed by Werner and Seiber (1963), may be
involved.
It has also been reported that intestinal microflora may degrade methylamine
(Iyer and Kailio 1958). Dar et al (1985) found that pretreatment of rats with
neomycin to reduce bacterial microorganisms resulted in only a slight inhibition of
14C expiration during the initial 6 h following methylamine administration. These
results indicate that, at least in the rat, bacterial oxidation of methylamine in the
intestine is negligible.
Storage
Methylamine is stored in a cool, well-ventilated noncombustible area separatedfrom possible sources of ignition andoxidizing substances and mercury. Itssolutions are stored in a flammable liquidstorage room or cabinet. The gas is shippedin steel cylinders or tank cars; the liquid isshipped in steel drums or tank cars.
Shipping
UN1061 Methylamine, anhydrous, Hazard Class: 2.1; Labels: 2.1-Flammable gas. UN1235 Methylamine, aqueous solution, Hazard Class: 3; Labels: 3-Flammable liquid, 8-Corrosive material. Cylinders must be transported in a secure upright position, in a well-ventilated truck. Protect cylinder and labels from physical damage. The owner of the compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas cylinders without the express written permission of the owner.
Purification Methods
Dry the amine with sodium or BaO. It is commercially available in metal cylinders. [Beilstein 4 IV 118.]
Toxicity evaluation
The corrosive nature of methylamine produces irritation effects at all points of contact which is largely responsible for its toxic actions. Using radiotracer techniques, labeled macromolecules were fragmented and the formaldehyde generated interacts with proteins in vivo suggesting a risk factor for initiation of endothelial injury and subsequent atherosclerosis. Increased semicarbazide-sensitive amine oxidase catalyzes the conversion of methylamine to formaldehyde and increased activity has been found in patients with diabetes mellitus, chronic heart failure, and cerebral infarct and is associated with obesity. The deamination of methylamine may contribute to protein deposition, the formation of plaques, and inflammation and be may be involved in the pathophysiology of chronic vascular and neurologic disorders such as diabetes, atherosclerosis, and Alzheimer’s disease.
Incompatibilities
A medium-strong base. Reacts violently with strong acids; mercury, strong oxidizers; nitromethane. Corrosive to copper, zinc alloys; aluminum, and galvanized surfaces.
Waste Disposal
Return refillable compressed gas cylinders to supplier. Controlled incineration (incinerator equipped with a scrubber or thermal unit to reduce nitrogen oxides emissions).
Properties of Methylamine
| Melting point: | -93 °C(lit.) |
| Boiling point: | -6.3 °C(lit.) |
| Density | 0.785 g/mL at 25 °C |
| vapor density | 1.08 (20 °C, vs air) |
| vapor pressure | 27 psi ( 20 °C) |
| refractive index | n |
| Flash point: | 61 °F |
| storage temp. | Store below +30°C. |
| solubility | highly soluble in water (108g/100g) at 25°C; soluble in alcohol and
miscible with ether; HCl salt is soluble in water and absolute alcohol; compound
is insoluble in chloroform, acetone, ether, and ethyl acetate |
| form | Gas |
| pka | 10.63(at 25℃) |
| Specific Gravity | 0.901 (20℃/4℃) (40% Soln.) |
| PH | 14 (H2O, 20°C) |
| Odor Threshold | 0.035ppm |
| explosive limit | 4.9-20.8% |
| Water Solubility | Miscible with water, ethanol, benzene, acetone and ether. |
| Merck | 14,6014 |
| BRN | 741851 |
| Henry's Law Constant | (x 10-5 atm?m3/mol):
1.11 at 25 °C (Christie and Crisp, 1967) |
| Dielectric constant | 10.0(18℃) |
| Exposure limits | TLV-TWA 10 ppm (~12.3 mg/m3)(ACGIH,
MSHA, and OSHA); IDLH 100 ppm
(NIOSH). |
| Stability: | Stable. Highly flammable. Note wide explosion limits. Incompatible with oxidizing agents, acids, alkalies, alkaline earth metals, copper and its alloys, zinc and its alloys. |
| CAS DataBase Reference | 74-89-5(CAS DataBase Reference) |
| EPA Substance Registry System | Methylamine (74-89-5) |
Safety information for Methylamine
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H314:Skin corrosion/irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Methylamine
Methylamine manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![[1-(1,1'-BIPHENYL-4-YL)-3-PHENYL-1H-PYRAZOL-4-YL]METHYLAMINE](https://img.chemicalbook.in/StructureFile/ChemBookStructure8/GIF/CB0206712.gif)
![(1R)CYCLOPROPYL[3-METHOXY-4-(2-METHYLPROPOXY)PHENYL]METHYLAMINE](https://img.chemicalbook.in/StructureFile/ChemBookStructure9/GIF/CB5336997.gif)

![(1R)CYCLOPROPYL(3-[(4-NITROPHENYL)METHOXY]PHENYL)METHYLAMINE](https://img.chemicalbook.in/StructureFile/ChemBookStructure9/GIF/CB1221750.gif)
You may like
-
 Monomethylamine CASView Details
Monomethylamine CASView Details -
 Methylamine CAS 74-89-5View Details
Methylamine CAS 74-89-5View Details
74-89-5 -
 Methylamine CAS 74-89-5View Details
Methylamine CAS 74-89-5View Details
74-89-5 -
 Methylamine 2.0 M in THF CAS 74-89-5View Details
Methylamine 2.0 M in THF CAS 74-89-5View Details
74-89-5 -
 Methylamine CASView Details
Methylamine CASView Details -
 Mono Methyl Amine (MMA) 40%View Details
Mono Methyl Amine (MMA) 40%View Details
74-89-5 -
 Monomethylamine 40% (MMA 40%), For Industrial, Grade: TechnicalView Details
Monomethylamine 40% (MMA 40%), For Industrial, Grade: TechnicalView Details
74-89-5 -
 200 Kg Mono Methyl Amine, Industrial, Purity: 40%View Details
200 Kg Mono Methyl Amine, Industrial, Purity: 40%View Details
74-89-5
