Methyl valeraldehyde
Synonym(s):2-Methylvaleraldehyde
- CAS NO.:123-15-9
- Empirical Formula: C6H12O
- Molecular Weight: 100.16
- MDL number: MFCD00006986
- EINECS: 204-605-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
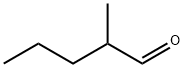
What is Methyl valeraldehyde ?
Description
2-Methylpentanal has an ethereal, fruity odor. This may be prepared from 2-methylpentanol by catalytic oxidation.
Chemical properties
Colorless liquid. Soluble in water 0.42% by wt, flash p68F (20C) (OC).
Occurrence
Reported found in onion, leek, garlic, milk, roasted peanuts, wheat bread, cooked beef, cured pork, coffee, beer, tea, trassi, rice and mango.
The Uses of Methyl valeraldehyde
Intermediates for dyes, resins, pharmaceuticals.
The Uses of Methyl valeraldehyde
2-Methylvaleraldehyde is used as an intermediate to manufacture organic compounds (pharmaceuticals, odorants and flavorings)
Preparation
From 2-methylpentanol by catalytic oxidation.
Definition
ChEBI: 2-Methylpentanal is a 2-methyl-branched fatty aldehyde.
Aroma threshold values
Detection: 1.6 to 3.2 ppb
Taste threshold values
Taste characteristics at 5.0 ppm: vegetative and green with a fruity, grape-like nuance.
Synthesis Reference(s)
Synthesis, p. 717, 1985 DOI: 10.1055/s-1985-31327
General Description
A colorless liquid. Less dense than water. Flash point near 50°F. Used to make rubber and artificial flavorings.
Air & Water Reactions
Highly flammable. Slightly soluble in water.
Reactivity Profile
Methyl valeraldehyde is an aldehyde. Aldehydes are frequently involved in self-condensation or polymerization reactions. These reactions are exothermic; they are often catalyzed by acid. Aldehydes are readily oxidized to give carboxylic acids. Flammable and/or toxic gases are generated by the combination of aldehydes with azo, diazo compounds, dithiocarbamates, nitrides, and strong reducing agents. Aldehydes can react with air to give first peroxo acids, and ultimately carboxylic acids. These autoxidation reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by the products of the reaction). The addition of stabilizers (antioxidants) to shipments of aldehydes retards autoxidation.
Hazard
Flammable, dangerous fire risk. Strong irri-tant to skin and mucous membranes.
Health Hazard
May cause toxic effects if inhaled or absorbed through skin. Inhalation or contact with material may irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Flammability and Explosibility
Highly flammable
Properties of Methyl valeraldehyde
| Melting point: | -100℃ |
| Boiling point: | 119-120 °C(lit.) |
| Density | 0.808 g/mL at 25 °C(lit.) |
| vapor density | 3.45 (vs air) |
| FEMA | 3413 | 2-METHYLPENTANAL |
| refractive index | n |
| Flash point: | 62 °F |
| solubility | Chloroform, Methanol (Slightly) |
| form | clear liquid |
| color | Colorless to Almost colorless |
| Odor | at 10.00 % in dipropylene glycol. ethereal fruity green |
| Water Solubility | Soluble in water (4.2g/L at 25°C). |
| Sensitive | Air Sensitive |
| JECFA Number | 260 |
| BRN | 1739423 |
| CAS DataBase Reference | 123-15-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Pentanal, 2-methyl-(123-15-9) |
| EPA Substance Registry System | Pentanal, 2-methyl- (123-15-9) |
Safety information for Methyl valeraldehyde
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Methyl valeraldehyde
Methyl valeraldehyde manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 123-15-9 2-Methylvaleraldehyde 98%View Details
123-15-9 2-Methylvaleraldehyde 98%View Details
123-15-9 -
 123-15-9 99%View Details
123-15-9 99%View Details
123-15-9 -
 2-Methylvaleraldehyde CAS 123-15-9View Details
2-Methylvaleraldehyde CAS 123-15-9View Details
123-15-9 -
 2-Methyl Pentanal CASView Details
2-Methyl Pentanal CASView Details -
 2-Methylpentanal CAS 123-15-9View Details
2-Methylpentanal CAS 123-15-9View Details
123-15-9 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8