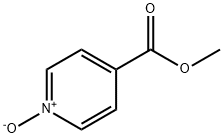
Methyl nicotinate
Synonym(s):Methyl nicotinate;Nicotinic acid methyl ester
- CAS NO.:93-60-7
- Empirical Formula: C7H7NO2
- Molecular Weight: 137.14
- MDL number: MFCD00006388
- EINECS: 202-261-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-02-14 15:59:18
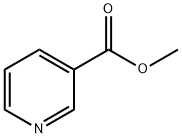
What is Methyl nicotinate?
Absorption
The presence of methyl group facilitates the penetration of methyl nicotinate through the skin with good lipophilicity, allowing rapid absorption following topical administration . In vitro, about 80-90% of the polar compounds methyl nicotinate rapidly penetrated the skin . It was demonstrated in excised skin of hairless mice that methyl nicotinate can effectively bypass the stratum corneum layer of the skin . In humans, nicotinic acid and nicotinamide were shown to be rapidly absorbed from the stomach and intestine via a sodium carrier-mediated mechanism at low concentrations .
Toxicity
The acute subcutaneous LD50 of structurally-related nicotinamide and nicotinic acid in rats were 1.68 and 5.0 g/kg bw, respectively . In humans, oral ingestion of 3-9 g of nicotinic acid per day resulted in "niacin hepatitis", gout, and impaired glucose intolerance within a short period of time .
Description
Methyl nicotinate is the methyl ester of Niacin that is used as an active ingredient as a rubefacient in over-the-counter topical preparations indicated for muscle and joint pain. The action of methyl nicotinate as a rubefacient is thought to involve peripheral vasodilation. For veterinary purposes, methyl nicotinate is used to treat respiratory diseases, vascular disorders, rheumatoid arthritis, and muscle and joint pains.
Chemical properties
Methyl nicotinate is white to brownish crystals or crystalline powder and has a fresh, caramellic, nutty and mild tobacco odor.
The Uses of Methyl nicotinate
Methyl nicotinate is used as an active ingredient, a rubefacient and counter-irritant in over-the-counter topical preparations indicated for the temporary relief of aches and pains in muscles,tendons, and joints. Methyl nicotinate can be found in in guava fruit, papaya, strawberry, soursop(Annona muricata), beer, grape brandy, coffee, roasted filbert, roasted peanut and Bourbon vanilla.
Indications
Indicated for the temporary relief of aches and pains in muscles, tendons, and joints.
Preparation
Methyl nicotinate was prepared by esterification of nicotinic acid by refluxing with methanol in the presence of concentrated sulphuric acid, esterification product obtained was extracted into organic solvent (chloroform) after neutralization of the reaction mixture with 10% sodium bicarbonate.
Synthesis and antinociceptive activity of methyl nicotinate
DOI:10.4314/jpb.v12i1.8
Pharmacokinetics
Following topical administration, methyl nicotinate acts as a peripheral vasodilator to enhance local blood flow at the site of application. It induced vasodilation of the peripheral blood capillaries which are located in the dermal papillae of upper dermis layers adjacent to the epidermis–dermis junction . During tissue penetration at the dermis, methyl nicotinate is hydrolyzed to nicotinic acid . In human volunteers, topical administration of methyl nicotinate caused vasodilation-induced generalized cutaneous erythema .
Side Effects
There is presently no side effect information available for Methyl nicotinate(MN) itself. a few patients who applied a medicated topical preparation containing MN (and other components) suffered adverse reactions including faintness, nausea, pain and skin rashes.
Metabolism
Methyl nicotinate undergoes ester hydrolysis to form nicotinic acid and methanol . The hydrolysis is thought to be mediated by nonspecific α-naphthylacetate-esterase at the dermis layer of the skin .
Properties of Methyl nicotinate
| Melting point: | 42-44 °C (lit.) |
| Boiling point: | 204 °C (lit.) |
| Density | 1.2528 (rough estimate) |
| vapor pressure | 0.4 hPa (25 °C) |
| FEMA | 3709 | METHYL NICOTINATE |
| refractive index | 1.5323 (estimate) |
| Flash point: | 204 °F |
| storage temp. | Store below +30°C. |
| solubility | H2O: 0.1 g/mL, clear |
| form | Crystals or Crystalline Powder |
| pka | pKa 3.1 (Uncertain) |
| color | White to brownish |
| Odor | at 10.00 % in dipropylene glycol. warm herbal tobacco |
| Water Solubility | NEGLIGIBLE |
| JECFA Number | 1320 |
| Merck | 14,6100 |
| BRN | 113951 |
| CAS DataBase Reference | 93-60-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Methyl nicotinate(93-60-7) |
| EPA Substance Registry System | Methyl nicotinate (93-60-7) |
Safety information for Methyl nicotinate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Methyl nicotinate
| InChIKey | YNBADRVTZLEFNH-UHFFFAOYSA-N |
Methyl nicotinate manufacturer
ALTRAKEM PHARMA LIFE SCIENCES PRIVATE LIMITED
VRUDDISRI PHARMA
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran
You may like
-
 Methyl nicotinate 93-60-7 98%View Details
Methyl nicotinate 93-60-7 98%View Details
93-60-7 -
 93-60-7 Methyl Nicotinate 98%View Details
93-60-7 Methyl Nicotinate 98%View Details
93-60-7 -
 Methyl nicotinate 98% CAS 93-60-7View Details
Methyl nicotinate 98% CAS 93-60-7View Details
93-60-7 -
 Methyl nicotinate 93-60-7 98%View Details
Methyl nicotinate 93-60-7 98%View Details
93-60-7 -
 93-60-7 Methyl nicotinate 98%View Details
93-60-7 Methyl nicotinate 98%View Details
93-60-7 -
 Methyl Nicotinate CAS 93-60-7View Details
Methyl Nicotinate CAS 93-60-7View Details
93-60-7 -
 Methyl nicotinate CAS 93-60-7View Details
Methyl nicotinate CAS 93-60-7View Details
93-60-7 -
 Methyl nicotinate, 99% CAS 93-60-7View Details
Methyl nicotinate, 99% CAS 93-60-7View Details
93-60-7
