Fluorescein isothiocyanate isomer I
Synonym(s):FITC;Fluorescein isothiocyanate;Fluorescein 5-isothiocyanate;FITC, Isomer I;Fluorescein Isothiocyanate, Isomer I
- CAS NO.:3326-32-7
- Empirical Formula: C21H11NO5S
- Molecular Weight: 389.38
- MDL number: MFCD00005063
- EINECS: 222-042-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 13:37:16
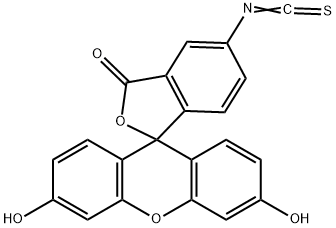
What is Fluorescein isothiocyanate isomer I?
Description
Fluorescein 5-isothiocyanate (5-FITC) is an isomer of fluorescein isothiocyanate (FITC), which is commonly used as a mixture of the 5- and 6-isothiocyanate isomers. It reacts with amine and thiol groups to form conjugates with proteins, lipids, and other molecules for detection by a variety of fluorescent-based applications. 5-FITC displays excitation/emission maxima of 494/519 nm, respectively.
Chemical properties
Fluorescein isothiocyanate(3326-32-7) is an orange-yellow crystal that is hygroscopic. It can be combined with various antibody proteins, and the combined antibody does not lose its specificity for binding to a certain antigen, and still has strong green fluorescence in alkaline solution, precipitates out after adding acid, and the fluorescence disappears. It is slightly soluble in acetone and ether and petroleum ether.
The Uses of Fluorescein isothiocyanate isomer I
Fluorescein isothiocyanate isomer I has been used for the staining of conidia.
The Uses of Fluorescein isothiocyanate isomer I
Fluorescein isothiocyanate isomer I has been used for the staining of conidia. It is a kind of fluorescent marker for biochemical applications. Reacts under mild conditions with primary amines and it is also used in modification of actin at lys-61, labelling of FAB and the modification of thiol groups.
What are the applications of Application
Fluorescein isothiocyanate is used as fluorescent labeling reagent for proteins and for rapid identification of pathogens in fluorescent antibody technique. It is also used as a reagent for microsequencing of proteins and peptides (HPLC). On celite it is used for the labeling of fibrinogen.
What are the applications of Application
Fluorescein isothiocyanate isomer I is a compound for the FITC labeling of proteins
Definition
ChEBI: Fluorescein 5-isothiocyanate is the 5-isomer of fluorescein isothiocyanate. Acts as a fluorescent probe capable of being conjugated to tissue and proteins; used as a label in fluorescent antibody staining procedures as well as protein- and amino acid-binding techniques.
General Description
Fluorescein isothiocyanate (FITC) is yellow-orange in color with an absorption maximum at 495nm. Upon excitation, it emits a yellow-green color with an emission maximum at 525nm.
It is widely used to attach a fluorescent label to proteins via the amine group. The isothiocyanate group reacts with amino terminal and primary amines in proteins. It has been used for the labeling of proteins including antibodies and lectins.
Fluorescein isothiocyanate isomer I has been proposed as a contact sensitizer.
storage
Store at -20°C
Purification Methods
It is made from the pure 5-amino isomer. Purify it by dissolving it in boiling Me2CO, filtering and adding pet ether (b 60-70o) until it becomes turbid. If an oil separates, then decant it and add more pet ether to the supernatant and cool. Orange-yellow crystals separate, collect and dry them in vacuo. It should give one spot on TLC (silica gel) in EtOAc/pyridine/AcOH (50:1:1) and in Me2NCHO/CHCl3/28% NH4OH (10:5:4). IR (Me2SO): 2110 (NCS) and 1760 (C=O) cm-1. The max 1HNMR spectra in Me2CO-d6 of the 5-and 6-isomers are distinctly different for the protons in the *benzene ring; the UV in phosphate buffer pH 8.0 shows a at ~490nm. [Sinsheimer et al. Anal Biochem 57 2271974, McKinney et al. Anal Biochem 7 74 1964, Beilstein 19 III/IV 4337.]
Properties of Fluorescein isothiocyanate isomer I
| Melting point: | >360 °C(lit.) |
| Boiling point: | 708.6±60.0 °C(Predicted) |
| Density | 1.3423 (rough estimate) |
| refractive index | 1.5060 (estimate) |
| storage temp. | 2-8°C |
| solubility | Slightly soluble in DMSO, ethanol, methanol DMF and acetone |
| form | powder |
| pka | 2.2, 4.4, 6.7(at 25℃) |
| color | White |
| PH Range | Weak green uorescence (6.0) to strong green uorescence (7.2) |
| Water Solubility | practically insoluble |
| Sensitive | Moisture Sensitive |
| λmax | 490nm |
| BRN | 1407295 |
| Stability: | Stability Stable, but moisture-sensitive. Incompatible with strong oxidizing agents, strong bases, amines, acids. |
| Major Application | Magnetic nanowires, immunoassays, analyzing proteins, identifying chromosomes, Salmonella mosomes, diagnosis of cancer, kidney diseases, detecting pathogens, genes, Salmonella cells, toxoplasmosis, enzyme-mediated nucleic acid cleavage, surface molecules on colorectal cancers, treating cancer, chronic lymphocytic leukemia |
| Biological Applications | Analyzing sugar
chain; detecting iodide/iodate or total iodine;
detecting/imaging thiols; detecting/quantifying biotin
derivatives;16 diagnosing classical Hodgkin lymphoma
(CHL) in lymph nodes; cellulose nanocrystals for
bioimaging applications; treating neuroinflammation;
treating/diagnosing cancer; PEO-PCL-PEO triblock
copolymers for topical delivery; polymersomes in
biomedical or cosmetic applications;apoptosis assay;
as a substrate for measuring heparanase activity, human
immunodeficiency virus (HIV-1) proteinase activity,
kinase activity, phosphatase activity, β-lactamase
activity, transglutaminase (TG2) activity; implantable
drug-delivery devicess |
| CAS DataBase Reference | 3326-32-7(CAS DataBase Reference) |
| EPA Substance Registry System | Spiro[isobenzofuran-1(3H),9'-[9H]xanthen]-3-one, 3',6'-dihydroxy-5-isothiocyanato- (3326-32-7) |
Safety information for Fluorescein isothiocyanate isomer I
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H334:Sensitisation, respiratory |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P272:Contaminated work clothing should not be allowed out of the workplace. P280:Wear protective gloves/protective clothing/eye protection/face protection. P284:Wear respiratory protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P333+P313:IF SKIN irritation or rash occurs: Get medical advice/attention. |
Computed Descriptors for Fluorescein isothiocyanate isomer I
| InChIKey | MHMNJMPURVTYEJ-UHFFFAOYSA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![3',6'-Dihydroxy-6-isothiocyanatospiro[isobenzofuran-1(3H),9'-[9H]xanthen]-3-one](https://img.chemicalbook.in/CAS/GIF/3326-31-6.gif)
You may like
-
 Fluorescein isothiocyanate, isomer 1 CAS 3326-32-7View Details
Fluorescein isothiocyanate, isomer 1 CAS 3326-32-7View Details
3326-32-7 -
 Fluorescein isothiocyanate isomer I (HPLC) CAS 3326-32-7View Details
Fluorescein isothiocyanate isomer I (HPLC) CAS 3326-32-7View Details
3326-32-7 -
 Fluorescein Isothiocyanate Isomer I (FITC) extrapure CAS 3326-32-7View Details
Fluorescein Isothiocyanate Isomer I (FITC) extrapure CAS 3326-32-7View Details
3326-32-7 -
 Fluorescein isothiocyanate isomer I 95% CAS 3326-32-7View Details
Fluorescein isothiocyanate isomer I 95% CAS 3326-32-7View Details
3326-32-7 -
 Fluorescein isothiocyanate,10% CAS 3326-32-7View Details
Fluorescein isothiocyanate,10% CAS 3326-32-7View Details
3326-32-7 -
 Fluorescein 5-Isothiocyanate (isomer I) CAS 3326-32-7View Details
Fluorescein 5-Isothiocyanate (isomer I) CAS 3326-32-7View Details
3326-32-7 -
 Fluorescein isothiocyanate isomer I 95% (HPLC) CAS 3326-32-7View Details
Fluorescein isothiocyanate isomer I 95% (HPLC) CAS 3326-32-7View Details
3326-32-7 -
 Fluorescein isothiocyanate isomer I CAS 3326-32-7View Details
Fluorescein isothiocyanate isomer I CAS 3326-32-7View Details
3326-32-7