methyl ibogamine-18-carboxylate
- CAS NO.:467-77-6
- Empirical Formula: C21H26N2O2
- Molecular Weight: 338.448
- MDL number: MFCD11111278
- EINECS: 207-398-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-07-25 20:04:51
What is methyl ibogamine-18-carboxylate?
The Uses of methyl ibogamine-18-carboxylate
Ibogamine-18-carboxylic Acid Methyl Ester (CAS# 467-77-6) is a useful research chemical compound. It is used in the biosynthesis of anti-addiction agent from iboga plant.
Definition
ChEBI: (-)-coronaridine is a monoterpenoid indole alkaloid with formula C21H26N2O2. It is isolated from the flowering plant genus, Tabernaemontana. It has a role as an antileishmanial agent, an antineoplastic agent, an apoptosis inducer and a plant metabolite. It is a monoterpenoid indole alkaloid, a methyl ester, an organic heteropentacyclic compound and an alkaloid ester. It is a conjugate base of a (-)-coronaridine(1+).
Biological Activity
Coronaridine, an iboga type alkaloid, inhibits the wnt signaling pathway by decreasing β-catenin expression[1]. Coronaridine (0-40 μM; 24 hours) is against non-cancer cells with IC50 values >40 μM. It agaisnt wnt-dependent cells with IC50 values of 10.4, 11.6 and 24.4 μM for SW480, HCT116 and DLD1cells, respectively[1].Coronaridine (0-40 μM; 24 hours) inhibits β-catenin expression, but the protein levels of p-β--catenin at Ser33, Ser37, and Thr41 and p-β-catenin at Ser 45 [p-b-catenin (S45)] are unchanged[1].In whole-cell patch clamp recordings,Catharanthine (1-300 μM) are respectively co-applied with GABA at concentrations corresponding to the EC30 value for each receptor subtype. Both congeners potentiated different GABAARs in a concentration-dependent manner[2].At higher concentrations, however, Catharanthine starts to inhibit GABA-activated currents due to the reduced amplitude and rebound current, where the threshold concentration depended on the receptor subtype (e.g., > 30 μM for hα1β2; > 100 μM for hα1β2γ2 and hα2β2γ2). The PAM activity of Catharanthine's are depended on the receptor subtype: hα1β2 (4.6±0.8 μM), >hα2β2γ2 (12.6±3.8 μM), hα1β2γ2 (14.4 ± 4.6 μM)[2].
References
[1]. Kensuke Ohishi, et al. Coronaridine, an iboga type alkaloid from Tabernaemontana divaricata, inhibits the Wnt signaling pathway by decreasing β-catenin mRNA expression. Bioorg Med Chem Lett. 2015 Sep 15;25(18):3937-40. [2]. Hugo R Arias, et al. Coronaridine congeners potentiate GABA A receptors and induce sedative activity in mice in a benzodiazepine-insensitive manner. Prog Neuropsychopharmacol Biol Psychiatry. 2020 Jul 13;101:109930
Properties of methyl ibogamine-18-carboxylate
| Melting point: | 92-93 °C |
| Boiling point: | 488.1±45.0 °C(Predicted) |
| Density | 1.24±0.1 g/cm3(Predicted) |
| storage temp. | 4°C, protect from light |
| pka | 16?+-.0.60(Predicted) |
| form | Solid |
| color | White to off-white |
Safety information for methyl ibogamine-18-carboxylate
Computed Descriptors for methyl ibogamine-18-carboxylate
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

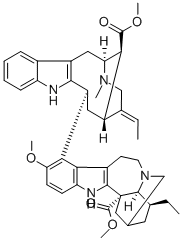
![13-Methoxy-14-[(3R)-17-methoxy-17-oxovobasan-3α-yl]ibogamine-18-carboxylic acid methyl ester](https://img.chemicalbook.in/CAS/GIF/2665-57-8.gif)
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1