METHYL FLUORIDE
- CAS NO.:593-53-3
- Empirical Formula: CH3F
- Molecular Weight: 34.03
- MDL number: MFCD00039282
- EINECS: 209-796-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
What is METHYL FLUORIDE?
Chemical properties
colourless gas with an ether-like odour
Definition
ChEBI: A member of the class of fluoromethanes that is methane in which a single hydrogen is substituted by a fluorine atom.
General Description
METHYL FLUORIDE (or fluoromethane) is a colorless flammable gas which is heavier than air. METHYL FLUORIDE has an agreeable ether-like odor. METHYL FLUORIDE is narcotic in high concentrations. METHYL FLUORIDE burns with evolution of hydrogen fluoride. The flame is colorless, similar to alcohol. Under prolonged exposure to fire or intense heat the containers may rupture violently and rocket.
Air & Water Reactions
Highly flammable. METHYL FLUORIDE burns in air with evolution of hydrogen fluoride.
Reactivity Profile
Halogenated aliphatic compounds, such as METHYL FLUORIDE, are moderately or very reactive. Reactivity generally decreases with increased degree of substitution of halogen for hydrogen atoms. Low molecular weight haloalkanes are highly flammable and can react with some metals to form dangerous products. Materials in this group are incompatible with strong oxidizing and reducing agents. Also, they are incompatible with many amines, nitrides, azo/diazo compounds, alkali metals, and epoxides. The prolonged mixing of halogenated solvents with metallic or other azides may cause the slow formation of explosive azides, for example methylene chloride and sodium azide, [Chem. Eng. News, 1986, 64(51)].
Hazard
Flammable. Narcotic in high concentrations.
Health Hazard
Vapors may cause dizziness or asphyxiation without warning. Some may be irritating if inhaled at high concentrations. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire may produce irritating and/or toxic gases.
Fire Hazard
EXTREMELY FLAMMABLE. Will be easily ignited by heat, sparks or flames. Will form explosive mixtures with air. Vapors from liquefied gas are initially heavier than air and spread along ground. CAUTION: Hydrogen (UN1049), Deuterium (UN1957), Hydrogen, refrigerated liquid (UN1966) and Methane (UN1971) are lighter than air and will rise. Hydrogen and Deuterium fires are difficult to detect since they burn with an invisible flame. Use an alternate method of detection (thermal camera, broom handle, etc.) Vapors may travel to source of ignition and flash back. Cylinders exposed to fire may vent and release flammable gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket.
Safety Profile
Narcotic in high concentrations. Acts as a simple asphyxiant. Burns with evolution of hydrogen fluoride. The flame is about as colorless as that of alcohol. When heated to decomposition it emits toxic fumes of F-.
Properties of METHYL FLUORIDE
| Melting point: | -115°C |
| Boiling point: | -79°C |
| Density | 0,877 g/cm3 |
| vapor pressure | 1965-5874kPa at 0-15℃ |
| refractive index | 1.1740 |
| Water Solubility | 1.787g/L(29.9 ºC) |
| Stability: | Stable. Extremely flammable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 593-53-3(CAS DataBase Reference) |
| EPA Substance Registry System | HFC-41 (593-53-3) |
Safety information for METHYL FLUORIDE
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Gas Cylinder Compressed Gases GHS04 |
| GHS Hazard Statements |
H220:Flammable gases H280:Gases under pressure H336:Specific target organ toxicity,single exposure; Narcotic effects |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for METHYL FLUORIDE
New Products
2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Pyridineacetonitrile, α-hydroxy- 3-Iodophenylacetic acid 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Strontium Carbonate, 98% Wang resin Sodium hydrogenphosphate, anhydrous 2-Bromo-3-methoxyaniline hydrochloride, 95% (Custom work) 1-Bromo-4-chlorobenzene, 99% Benzocaine, 98% (R)-2-Methylpyrolidine-2-carboxylic acid (De Mepro) Ramipril Sacubitril- Valsartan Boc-his(trt)-OH Fmoc-L-Glu-OtBu Boc-L-Tyr(tBu)-OH Semi carbazide Hydrochloride 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 2-Chloromethyl-4-methyl-quinazoline Trans-4-Aminocyclohexanol [4tac] 2-[1-(Mercaptomethyl)Cyclopropyl]Acetic AcidRelated products of tetrahydrofuran
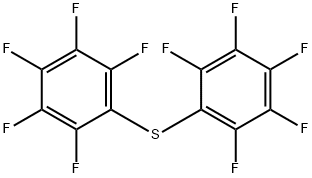
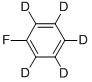
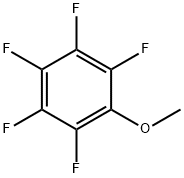
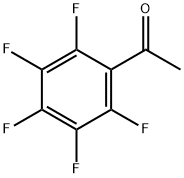
You may like
-
 15761-38-3 N-Boc-L-Alanine >98%View Details
15761-38-3 N-Boc-L-Alanine >98%View Details
15761-38-3 -
 2,4-Diamino 6-Chloro Pyrimidine 156-83-2 >98%View Details
2,4-Diamino 6-Chloro Pyrimidine 156-83-2 >98%View Details
156-83-2 -
 6485-34-3 Saccharin Calcium >98%View Details
6485-34-3 Saccharin Calcium >98%View Details
6485-34-3 -
 Boc-Gly-Gly-Phe-Gly-OH 187764-46-6 >98%View Details
Boc-Gly-Gly-Phe-Gly-OH 187764-46-6 >98%View Details
187764-46-6 -
 (S)-2-Methylpyrrolidine-2-carboxylic acid HCl (MePro) 1508261-86-6 >98%View Details
(S)-2-Methylpyrrolidine-2-carboxylic acid HCl (MePro) 1508261-86-6 >98%View Details
1508261-86-6 -
 Fmoc-Gly-Pro-OH >98%View Details
Fmoc-Gly-Pro-OH >98%View Details
212651-48-4 -
 18568-74-8 >98%View Details
18568-74-8 >98%View Details
18568-74-8 -
 138-41-0 P-Carboxy benzene sulfonamide >98%View Details
138-41-0 P-Carboxy benzene sulfonamide >98%View Details
138-41-0
