Methyl 4-formylbenzoate
Synonym(s):Methyl benzaldehyde-4-carboxylate;Methyl terephthalaldehydate
- CAS NO.:1571-08-0
- Empirical Formula: C9H8O3
- Molecular Weight: 164.16
- MDL number: MFCD00006950
- EINECS: 216-385-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-05-15 21:25:55
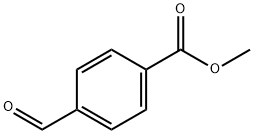
What is Methyl 4-formylbenzoate?
Chemical properties
white crystalline powder
The Uses of Methyl 4-formylbenzoate
Methyl 4-formylbenzoate is used in the preparation of dimethyl terephthalate. It is also used as an active pharmaceutical ingredient intermediate and fluorescent brightener intermediate.
The Uses of Methyl 4-formylbenzoate
4-Formylbenzoic Acid Methyl Ester is a benzoic acid derivative with antioxidant activity.
Synthesis Reference(s)
Tetrahedron, 64, p. 688, 2008 DOI: 10.1016/j.tet.2007.11.030
General Description
Needles (in water) or white powder.
Air & Water Reactions
Methyl 4-formylbenzoate may be sensitive to prolonged exposure to air. . Insoluble in water.
Reactivity Profile
Methyl 4-formylbenzoate is an aldehyde/ester. Aldehydes are frequently involved in self-condensation or polymerization reactions. These reactions are exothermic; they are often catalyzed by acid. Aldehydes are readily oxidized to give carboxylic acids. Flammable and/or toxic gases are generated by the combination of aldehydes with azo, diazo compounds, dithiocarbamates, nitrides, and strong reducing agents. Aldehydes can react with air to give first peroxo acids, and ultimately carboxylic acids. These autoxidation reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by the products of the reaction). The addition of stabilizers (antioxidants) to shipments of aldehydes retards autoxidation. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides.
Fire Hazard
Flash point data for Methyl 4-formylbenzoate are not available. Methyl 4-formylbenzoate is probably combustible.
Properties of Methyl 4-formylbenzoate
| Melting point: | 59-63 °C (lit.) |
| Boiling point: | 265 °C (lit.) |
| Density | 1,2 g/cm3 |
| refractive index | 1.5260 (estimate) |
| Flash point: | 144 °C |
| storage temp. | Store below +30°C. |
| solubility | methanol: 0.1 g/mL, clear |
| form | Crystalline Powder or Chunks |
| color | White to cream or slightly green |
| Water Solubility | insoluble |
| Sensitive | Air Sensitive |
| BRN | 775895 |
| Stability: | Stable, though possibly air-sensitive. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 1571-08-0(CAS DataBase Reference) |
| NIST Chemistry Reference | 4-HC(O)-C6H4-COOCH3(1571-08-0) |
| EPA Substance Registry System | Benzoic acid, 4-formyl-, methyl ester (1571-08-0) |
Safety information for Methyl 4-formylbenzoate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Methyl 4-formylbenzoate
| InChIKey | FEIOASZZURHTHB-UHFFFAOYSA-N |
Methyl 4-formylbenzoate manufacturer
SAKEM LLP
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Methyl Terephthalaldehydate CAS 1571-08-0View Details
Methyl Terephthalaldehydate CAS 1571-08-0View Details
1571-08-0 -
 Methyl 4-formylbenzoate 98% CAS 1571-08-0View Details
Methyl 4-formylbenzoate 98% CAS 1571-08-0View Details
1571-08-0 -
 Methyl 4-formylbenzoate CAS 1571-08-0View Details
Methyl 4-formylbenzoate CAS 1571-08-0View Details
1571-08-0 -
 Methyl 4-formylbenzoate CAS 1571-08-0View Details
Methyl 4-formylbenzoate CAS 1571-08-0View Details
1571-08-0 -
 Methyl-4-formylbenzoate 1571-08-0 98%View Details
Methyl-4-formylbenzoate 1571-08-0 98%View Details
1571-08-0 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
