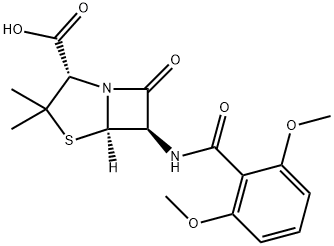
Methicillin Sodium Salt
Synonym(s):Sodium (2,6-dimethoxyphenyl)penicillin;Sodium methicillin
- CAS NO.:132-92-3
- Empirical Formula: C17H19N2NaO6S
- Molecular Weight: 402.39733
- MDL number: MFCD07787409
- EINECS: 205-083-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
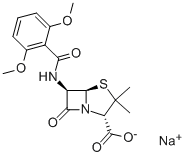
What is Methicillin Sodium Salt?
Description
Methicillin is a semisynthetic penicillin antibiotic that can inhibit bacterial cell wall synthesis. It can be used to study methicillin-resistance in S. aureus.
The Uses of Methicillin Sodium Salt
Targets primarily the cell wall of Gram-positive organisms especially Staphylococcus aureus. Methicillin is used to study bacterium susceptibility, and to inhibit cell-wall synthesis. PBP's are enzymes which catalyze a pentaglycine crosslink between alanine and lysine residues providing additional strength to the cell wall. Without a pentaglycine crosslink, the integrity of the cell wall is severely compromised and ultimately leads to cell lysis and death. Methicillin Sodium Salt is inactivated by gastric acid so it is given to cells by other means in which gastric acid is not present in vitro.
The Uses of Methicillin Sodium Salt
Semi-synthetic antibiotic related to Penicillin. Antimicrobial.
The Uses of Methicillin Sodium Salt
antibacterial
What are the applications of Application
Methicillin Sodium Salt is a semi-synthetic antibiotic related to penicillin
Definition
ChEBI: Methicillin sodium is an organic sodium salt. It contains a methicillin(1-).
brand name
Staphcillin (Apothecon).
Clinical Use
During 1960, methicillin sodium, 2,6-dimethoxyphenylpenicillinsodium (Staphcillin), the second penicillin produced asa result of the research that developed synthetic analogs, wasintroduced for medicinal use.
Methicillin sodium is particularly resistant to inactivationby the penicillinase found in staphylococci and somewhatmore resistant than penicillin G to penicillinase fromBacillus cereus.
in vitro
similar to other β-lactam antibiotics, meticillin acts via inhibiting the synthesis of bacterial cell walls. meticillin can block the cross-linkage between the linear peptidoglycan polymer chains by binding to and competitively inhibiting the transpeptidase enzyme or penicillin-binding proteins [1].
in vivo
in a previous animal study, the treatment with methicillin or gentamicin or both was started 3 days after infection to a experimental mouse model of foreign body infection. results found that the treatment showed a significant effect, demonstrated as reduction of bacteria on the foreign body, for all three regimens with a reduction of up to 2 log units, but there was no synergism. however, the actual efficacy of the treatment was poor, though the local methicillin concentrations was greater than the mic for at least 72 h [2].
References
[1] https://en. wikipedia.org/wiki/meticillin
[2] espersen f, frimodt-m ller n, corneliussen l, riber u, rosdahl vt, skinh j p. effect of treatment with methicillin and gentamicin in a new experimental mouse model of foreign body infection. antimicrob agents chemother. 1994 sep;38(9):2047-53.
Properties of Methicillin Sodium Salt
| Melting point: | 196-197℃ |
| Boiling point: | 640℃ |
| Flash point: | >110°(230°F) |
| storage temp. | 2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly), Water (Slightly) |
| form | powder |
| color | white to off-white |
| Water Solubility | Slightly soluble in acetone. Soluble in ethanol or water |
| Sensitive | Light Sensitive |
Safety information for Methicillin Sodium Salt
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H334:Sensitisation, respiratory H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Methicillin Sodium Salt
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Methicillin sodium salt CAS 132-92-3View Details
Methicillin sodium salt CAS 132-92-3View Details
132-92-3 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8