melarsoprol
- CAS NO.:494-79-1
- Empirical Formula: C12H15AsN6OS2
- Molecular Weight: 398.345
- MDL number: MFCD00866803
- EINECS: 2077934
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-07-15 16:45:23
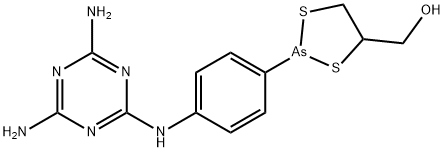
What is melarsoprol ?
Description
Knowingly or unknowingly, arsenic-containing drugs have been used for treatment of parasitic conditions for thousands of years. In the late 1800s and early 1900s, Paul Ehrlich introduced the use of trivalent arsenicals. Melarsoprol, an organoarsenical, came into use in the late 1940s, and it remains the first-choice drug in the treatment of trypanosomiasis. Until 1990, it also was the only treatment for late-stage sleeping sickness.
The Uses of melarsoprol
Melarsoprol is a drug used for the treatment of African trypanosomes, a sleeping sickness in humans, a disease that is typically fatal without chemotherapy.
Definition
ChEBI: Melarsoprol is a member of triazines.
Antimicrobial activity
It is highly and rapidly active against Trypanosoma brucei gambiense and T. brucei rhodesiense in vitro at submicromolar concentrations. It is much less active against the trypanosomes that infect domestic animals, T. congolense and T. vivax. Co-administration with eflornithine is effective against central nervous system (CNS) infection with T. brucei in rodent models, but clinical studies have found the combination less effective than nifurtimox–eflornithine.
Acquired resistance
Up to 25% of cases of T. brucei gambiense in Central Africa relapse. Patients infected with T. brucei rhodesiense normally respond to a second course of the drug, but those with T. brucei gambiense do not. In laboratory-generated resistant strains, decreased sensitivity results from reduced uptake of the drug by bloodstream trypomastigotes that either lack an adenine/adenosine transporter (TbAT1) or contain a transporter gene with point mutations. There is conflicting evidence about the role of this mechanism of resistance in isolates from patients unresponsive to treatment.
Health Hazard
Melarsoprol (4, Mel B, Arsobal C12H15AsN6OS2) is applied for T. b. gambiense or T. b. rhodesiense. The drug, administered intravenously, is a solution containing a combination of BAL (2,3-dimercaptopropanol) and the trivalent arsenic compound, melarsen oxide. Not only can the drug cause serious side effects such as intense dermal irritation, myocarditis, and renal and hepatic damage, but it is also responsible for death in 5% of patients.
Pharmaceutical Applications
Mel B. A derivative of trivalent melarsen oxide and dimercaprol (BAL), possessing a melaminyl moiety. Formulated in 3.6% propylene glycol for intravenous administration. It is almost insoluble in water.
Mechanism of action
It generally is accepted that the enzyme with which melarsoprol reacts is an enzyme involved in glycolysis, and as a result, inhibition of pyruvate kinase occurs. It is argued, however, that the inhibition may not occur at pyruvate kinase but, rather, at a step before the pyruvate kinase. Blockage of glycolysis would be expected to lead to loss of motility and cell lysis. More recently, Fairlamb et al. have proposed a mechanism of action that results in the inhibition of trypanothione reductase through the formation of a stable complex between melaroprol and trypanothione. Melarsoprol reacts with the cysteine sulfhydryl of trypanothione to form the stable adduct shown in Figure 39.10. Supportive of this mechanism is the synergistic action of melarsoprol with eflornithine (DMFO). Two drugs that produce sequential blockage of the synthesis of trypanothione.
Pharmacokinetics
Serum levels of 2–4 mg/L were achieved 24 h after administration of 3.6 mg/kg, falling to 0.1 mg/L at 120 h after the fourth daily injection. Elimination was biphasic with a half-life of 35 h. The volume of distribution was 100 L. It is rapidly metabolized by microsomal enzymes to melarsen oxide, reaching maximum plasma concentration by 15 min and eliminated with a half-life of 3.9 h. This metabolite can cross the blood–brain barrier and effect a CNS cure in mice. Levels of melarsoprol in the cerebrospinal fluid (CSF) reached around 300 μg/L, about 50 times lower than serum levels.
Clinical Use
Late-stage sleeping sickness caused by T. brucei gambiense and T. brucei
rhodesiense
It is not recommended for early-stage disease, in which alternatives
with less serious side effects are available.
Clinical Use
2-p-(4,6-Diamino-s-triazin-2-yl-amino)phenyl-4-hydroxymethyl-1,3,2-dithiarsoline (Mel B, Arsobal) is prepared byreduction of a corresponding pentavalent arsanilate to thetrivalent arsenoxide followed by reaction of the latter with2,3-dimercapto-1-propanol (British anti-Lewisite [BAL]). Ithas become the drug of choice for the treatment of thelater stages of both forms of African trypanosomiasis.Melarsoprol has the advantage of excellent penetration intothe CNS and, therefore, is effective against meningoencephaliticforms of T. gambiense and T. rhodesiense.Trivalent arsenicals tend to be more toxic to the host (as wellas the parasites) than the corresponding pentavalent compounds.The bonding of arsenic with sulfur atoms tends toreduce host toxicity, increase chemical stability (to oxidation),and improve distribution of the compound to the arsenoxide.Melarsoprol shares the toxic properties of other arsenicals, however, so its use must be monitored for signsof arsenic toxicity.
Side Effects
The propylene glycol formulation can cause tissue trauma and long-term damage to veins. Drug-induced reactions include fever on first administration, abdominal colic pain, dermatitis and arthralgia. Polyneuropathy has been reported in about 10% of patients. Reactive arsenical encephalopathy is a serious side effect that occurs in around 10% of those treated, with death in 1–3% of cases. The frequency of encephalopathy increases with a rise in the white cell count or the presence of trypanosomes in the CSF. The causes of the immunological responses involved in the encephalopathy and the possible existence of two forms (reactive and hemorrhagic) are not completely resolved. Studies to identify antiinflammatory approaches to reduce reactive encephalopathy in late-stage T. brucei gambiense infection have produced limited results.
Metabolism
Melarsoprol is administered IV in multiple doses and multiple sessions. Its major metabolite in humans is the lipophilic melarsen oxide, which can penetrate into the CNS. This metabolite apparently is responsible for the protein-binding characteristic for melarsoprol.
Properties of melarsoprol
| solubility | DMSO (Slightly), Methanol (Slightly, Heated) |
| form | Solid |
| color | White to Off-White |
Safety information for melarsoprol
Computed Descriptors for melarsoprol
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 6-Bromo-3-iodo-1-methyl-1H-indazole N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine N-Boc-L-proline methyl ester 2-(BOC-Amino)4-picoline 1-(4-Methylphenylsulfonyl)-1H-1,2,3-benzotriazole 1-(2-Chlorobenzyl)-4-nitro-1H-pyrazoleRelated products of tetrahydrofuran

You may like
-
 Melarsoprol CAS 494-79-1View Details
Melarsoprol CAS 494-79-1View Details
494-79-1 -
 55441-95-7 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 -
 Ste-Glu-AEEA-AEEA-OSUView Details
Ste-Glu-AEEA-AEEA-OSUView Details
1169630-40-3 -
 1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 -
 127464-43-1 99%View Details
127464-43-1 99%View Details
127464-43-1 -
 Chloro Uracil 99%View Details
Chloro Uracil 99%View Details
1820-81-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 13162-05-5 99%View Details
13162-05-5 99%View Details
13162-05-5