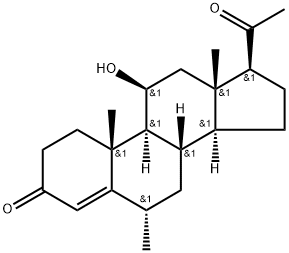
MEDRYSONE
- CAS NO.:2668-66-8
- Empirical Formula: C22H32O3
- Molecular Weight: 344.5
- MDL number: MFCD00056477
- EINECS: 220-208-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-09 19:38:33
What is MEDRYSONE?
Absorption
Rapidly absorbed following oral administration.
Description
6α-methyl-11β-hydroxy Progesterone is an anti-inflammatory steroid. It is a hydroxylated progesterone that lacks progestational and androgenic activity. It inhibits phytohemagglutinin-activated T cell proliferation in vitro with an IC50 value of 2.9 μg/ml. 6α-methyl-11β-hydroxy Progesterone also inhibits TNF-α production in LPS-stimulated THP-1 cells (IC50 = 30.54 μg/ml). Topical formulations containing 6α-methyl-11β-hydroxyprogesterone have been used to treat inflammatory ocular diseases.
Originator
HMS,Allergan,US,1970
The Uses of MEDRYSONE
Medrysone is a corticosteroid that is seen to increase intraocular pressure.
The Uses of MEDRYSONE
glucocorticoid
Background
Medrysone is a corticosteroid used in ophthalmology.
Indications
For the treatment of allergic conjunctivitis, vernal conjunctivitis, episcleritis, and epinephrine sensitivity.
What are the applications of Application
6α-Methyl-11β-hydroxyprogesterone is a corticosteroid and anti-inflammatory
Definition
ChEBI: Medrysone is a corticosteroid hormone.
Manufacturing Process
Preparation of 11-Keto-6β-Methylprogesterone 3,20-bis-(Ethylene Ketal): A
mixture of 5 g of 11-keto-6β-methylprogesterone [Spero et al, A Am. Chem.
Soc., 78, 6213 (1956)], 503 ml of benzene, 26 ml of ethylene glycol, and
0.152 g of p-toluenesulfonic acid monohydrate was stirred and heated under
reflux for 22 hours while water was removed by means of a water trap. The
reaction mixture was then cooled to 30°C, 0.4 ml of pyridine was added, and
stirring was continued for 10 minutes.
The reaction mixture was then shaken with 110 ml of water and the organic
and aqueous layers separated. The organic layer was dried over sodium
sulfate and evaporated under diminished pressure giving a residue. The thus
obtained residue was recrystallized from methanol giving 2.68 g of 11-keto6β-methyl progesterone 3,20-bis-(ethylene ketal) having a MP of 168° to
175°C.
Preparation of 11β-Hydroxy-6α-Methylprogesterone: A mixture of 2.68 g of 11-keto-6β-methylprogesterone 3,20-bis-(ethylene ketal), 161 ml of
tetrahydrofuran (previously distilled from lithium aluminum hydride), 1.34 g of
lithium aluminum hydride and 14.5 ml of absolute ether was stirred and
refluxed under nitrogen for 1.5 hours, then 27 ml of water was added
cautiously, to decompose excess hydride. The resulting mixture was filtered
and the filter cake was washed with 135 ml of ether. The combined filtrate
and wash was shaken with 135 ml of water and separated. The aqueous layer
was washed with four 55-ml portions of ether, then the organic layer and the
washes were combined, washed once with water, and evaporated to dryness
under diminished pressure leaving a tan residue.
The thus-obtained residue was dissolved in a mixture of 268 ml of methanol
and 26.8 mi of 3 N aqueous sulfuric acid and heated under reflux for 40
minutes, with a color change from yellow to green. The reaction mixture was
then cooled, neutralized by addition of 127 ml of 5% sodium bicarbonate
solution, and concentrated under reduced pressure until almost all the
methanol was removed. The resulting solid was removed by filtration, washed
with water, dried, and twice crystallized from ethyl acetate to give 1.1 g of
11β-hydroxy-6α-methylprogesterone having a MP of 155° to 158°C, according
to US Patent 2,864,837.
Therapeutic Function
Glucocorticoid
Pharmacokinetics
Medrysone is a topical anti-inflammatory corticoidsteroids for ophthalmic use. In patients with increased intraocular pressure and in those susceptible to a rise in intraocular pressure, there is less effect on pressure with medrysone than with dexamethasone or betamethasone. Corticoidsteroids inhibit the edema, fibrin deposition, capillary dilation, and phagocytic migration of the acute inflammatory response, as well as capillary proliferation, deposition of collagen, and scar formation.
Metabolism
Not Available
References
[1] the dictionary of drugs: chemical data: chemical data, structures and bibliographies[m]. springer, 2014.
[2] spaeth g l. hydroxymethylprogesterone: an anti-inflammatory steroid without apparent effect on intraocular pressure[j]. archives of ophthalmology, 1966, 75(6): 783-787.
Properties of MEDRYSONE
| Melting point: | 155-158° |
| Boiling point: | 492.3±45.0 °C(Predicted) |
| alpha | D +189° (in chloroform) |
| Density | 1.13±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | Acetone (Slightly), Chloroform (Slightly), Methanol (Slightly, Sonicated) |
| form | Solid |
| pka | 14.53±0.70(Predicted) |
| color | White to Off-White |
Safety information for MEDRYSONE
Computed Descriptors for MEDRYSONE
New Products
ALUMINIUM IODIDE 100 GM BUFFER CAPSULE PH 7.0 - 10 CAP BUFFER SOLUTION PH 9.5 (BORATE) EZEE BLUE GEL STAINER BORAX CARMINE (GRENACHERS ALCOHOLIC) POTASSIUM IODATE - IODIDE SOLN 0.1 N Dabigatran Acyl-O3-D-Glucuronide Trifluoroacetic Acid Salt Isofolic Acid Dabigatran 2-O-acylglucuronide metabolite Dabigatran Acyl-?-D- glucuronide Trifluroacetic Acid Erythromycin EP Impurity A Desloratidine Related Compound ARelated products of tetrahydrofuran



You may like
-
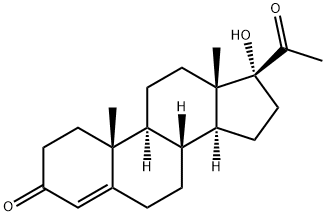
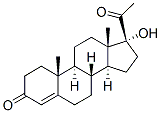
 6α-Methyl-11β-hydroxyprogesterone CAS 2668-66-8View Details
6α-Methyl-11β-hydroxyprogesterone CAS 2668-66-8View Details
2668-66-8 -
 Dechloro DesloratadineView Details
Dechloro DesloratadineView Details -
 Dehydro DesloratadineView Details
Dehydro DesloratadineView Details -
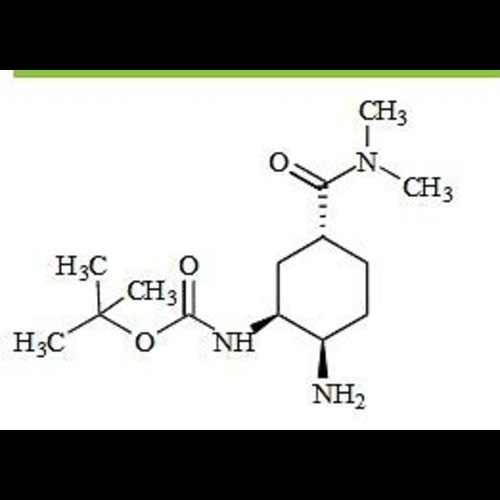 Edoxaban Impurity 57View Details
Edoxaban Impurity 57View Details
2089454-69-1 -
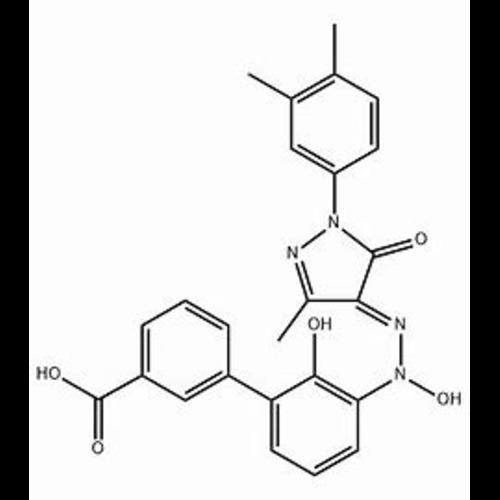 Eltrombopag N-Oxide ImpurityView Details
Eltrombopag N-Oxide ImpurityView Details
2734533-17-4 -
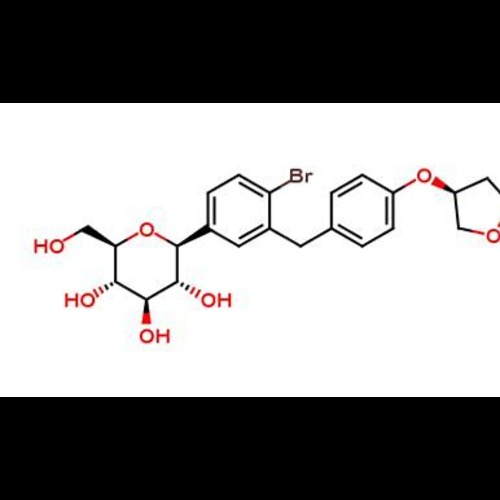 Empagliflozin Bromo ImpurityView Details
Empagliflozin Bromo ImpurityView Details -
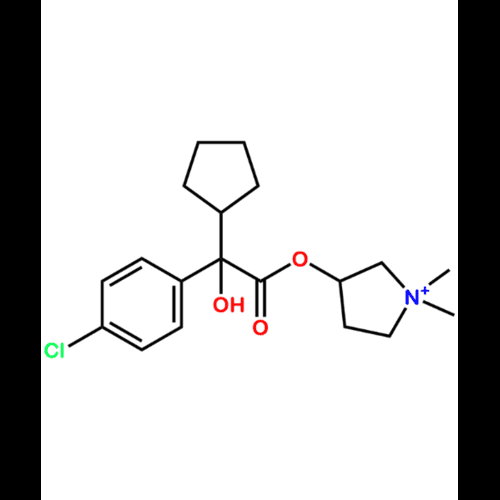 Glycopyrronium Bromide EP Impurity IView Details
Glycopyrronium Bromide EP Impurity IView Details
1404617-94-2 -
 Ipratropium EP Impurity BView Details
Ipratropium EP Impurity BView Details
58073-59-9