Medetomidine
- CAS NO.:86347-14-0
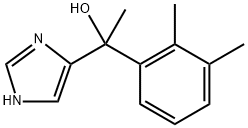
- Empirical Formula: C13H16N2
- Molecular Weight: 200.28
- MDL number: MFCD00864346
- EINECS: 1592732-453-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-11-24 17:44:56
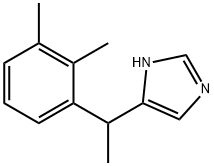
What is Medetomidine?
Description
Medetomidine is a synthetic compound used as a surgical anesthetic and analgesic. It is normally found as its hydrochloride salt, medetomidine hydrochloride. Medetomidine is an intravenously available alpha-2 adrenergic agonist. The drug has been developed by Orion Pharma. In the United States, it is currently approved for its veterinary use in dogs and distributed by Pfizer Animal Health. In Canada, medetomidine is distributed by Novartis Animal Health. The marketed product is a racemic mixture of two stereoisomers from which dexmedetomidine is the main active isomer.
The Uses of Medetomidine
Medetomidine is used in method for synthesizing Medetomidine using 2,3-Dimethylphenylmagnesium Bromide.
The Uses of Medetomidine
Analgesic (veterinary); sedative (veterinary).
Indications
Medetomidine Hydrochloride is indicated for use as a sedative and analgesic in dogs over 12 weeks of age to facilitate clinical examinations, clinical procedures, minor surgical procedures not requiring muscle relaxation, and minor dental procedures where intubation is not required. The IV route of administration is more efficacious for dental care.
Definition
ChEBI: Medetomidine is a member of imidazoles.
Clinical Use
Medetomidine is a potent non-narcotic α2-adrenoreceptor agonist which produces sedation and analgesia. These effects are dose dependent in depth and duration. Profound sedation and recumbency, with reduced sensitivity to environmental stimuli (sounds, etc.), are seen with medetomidine.
Side Effects
Bradycardia with occasional atrioventricular blocks will occur together with decreased respiratory rates. Body temperature is slightly or moderately decreased. Urination typically occurs during recovery at about 90 to 120 minutes posttreatment. In approximately 10% of treated dogs, occasional episodes of vomiting occur between 5 to 15 minutes posttreatment. An increase in blood glucose concentration is seen due to α2-adrenoreceptor-mediated inhibition of insulin secretion.
Veterinary Drugs and Treatments
Medetomidine is labeled for use as a sedative and analgesic in dogs over 12 weeks of age to facilitate clinical examinations and procedures, minor surgical procedures not requiring muscle relaxation, and minor dental procedures not requiring intubation. The manufacturer recommends the IV route of administration for dental procedures. Medetomidine has also been used in cats, primarily in Europe. But there is apparently much less data available to evaluate its use; caution is advised.
Precautions
In extremely nervous or excited dogs, levels of endogenous catecholamines are high due to the animal’s state of agitation. The pharmacological response elicited by α2-agonists (e.g., medetomidine) in such animals is often reduced, with depth and duration of sedative/analgesic effects ranging from slightly diminished to nonexistent. Highly agitated dogs should therefore be put at ease and allowed to rest quietly prior to receiving Medetomidine Hydrochloride. Allowing dogs to rest quietly for 10 to 15 minutes after injection may improve the response to Medetomidine Hydrochloride. In dogs not responding satisfactorily to treatment with Medetomidine Hydrochloride, repeat dosing is not recommended.
Properties of Medetomidine
| Boiling point: | 381.9±11.0 °C(Predicted) |
| Density | 1.053 |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Soluble in DMSO |
| form | Powder |
| pka | 14.29±0.10(Predicted) |
| color | White to off-white |
| InChI | InChI=1S/C13H16N2/c1-9-5-4-6-12(10(9)2)11(3)13-7-14-8-15-13/h4-8,11H,1-3H3,(H,14,15) |
Safety information for Medetomidine
Computed Descriptors for Medetomidine
| InChIKey | CUHVIMMYOGQXCV-UHFFFAOYSA-N |
| SMILES | C1NC(C(C2=CC=CC(C)=C2C)C)=CN=1 |
Medetomidine manufacturer
ALS India Life Sciences Pvt. Ltd
New Products
tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-Arg-OH HCl H2O Indole Methyl Resin 2-CTC Resin Gabapentin EP Impurity A 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 1,3-Diphenylurea 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID N-METHYL INDAZOLE-3-CARBOXYLIC ACID 2-Hydroxy-5-nitroacetophenone 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2-HYDROXY BENZYL CYANIDE DIETHYL AMINOMALONATE HYDROCHLORIDE 5-BROMO-2CYANO PYRIDINE Boldenone Clarithromycin Ethinyl Estradiol StanozololRelated products of tetrahydrofuran
You may like
-
 Medetomidine 95% CAS 86347-14-0View Details
Medetomidine 95% CAS 86347-14-0View Details
86347-14-0 -
 86347-14-0 98.42%View Details
86347-14-0 98.42%View Details
86347-14-0 -
 CIS BROMO BENZOATE 98%View Details
CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 90-01-7 2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 3-NITRO 2-METHYL BENZOIC ACID 98%View Details
3-NITRO 2-METHYL BENZOIC ACID 98%View Details
1975-50-4 -
 221615-75-4 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 98%View Details
221615-75-4 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 98%View Details
221615-75-4 -
 2033-24-1 MELDRUMS ACID 98%View Details
2033-24-1 MELDRUMS ACID 98%View Details
2033-24-1 -
 42831-50-5 98%View Details
42831-50-5 98%View Details
42831-50-5
