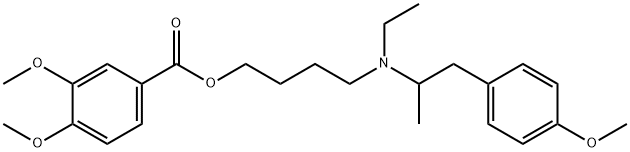
Mebeverine hydrochloride
- CAS NO.:2753-45-9
- Empirical Formula: C25H35NO5.ClH
- Molecular Weight: 466.01
- MDL number: MFCD00083411
- EINECS: 220-400-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
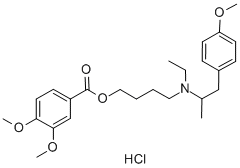
What is Mebeverine hydrochloride?
Chemical properties
White Solid
Originator
Duspatalin,Duphar,France,1965
The Uses of Mebeverine hydrochloride
Smooth muscle relaxant. Antispasmodic.
The Uses of Mebeverine hydrochloride
muscarinic ACh agonist, antiglaucoma
Manufacturing Process
(A) Sodium-3,4-Dimethoxybenzoate: A solution of 91 g of 3,4- dimethoxybenzoic acid in 500 ml of boiling, absolute alcohol was added quickly to a solution of 11.5 g of sodium in 300 ml of absolute alcohol; after cooling to room temperature the resulting precipitate was filtered off and washed with 2 x 50 ml of absolute alcohol and 4 x 200 ml of ether and dried in air to constant weight; yield 92.5 g, MP about 265°C. The filtrate was bulked with the alcohol and ether washings, left to stand overnight, and a further precipitate then filtered off, washed with 3 x 100 ml of ether, and dried in air to constant weight. Yield 22.5 g, MP about 265°C. Total yield therefore 115 g (=113%).
(B) 4'-Chlorobutyl-3,4-Dimethoxybenzoate: 92 g of the sodium salt described
under (A) (it contains at the most 81.5 g of sodium 3,4-dimethoxybenzoate)
was boiled in 900 ml of tetramethylene dichloride for 90 hours; after cooling
the mixture was filtered and the residue washed with 3 x 50 ml of ether. The
filtrate was evaporated to dryness in vacuo and the residue (102 g) was
distilled in vacuo. Fraction 1: 50° to 55°C/0.5 mm; 19 g (probably
tetramethylene dichloride). Fraction 2: 175° to 184°C/0.5 mm; 77.5 g
(=71%); Cl= 12.6% (calculated 13.0%). Remark: The second fraction
partially solidified or became more viscous on standing, and even during the
distillation.
(C) 4'-Iodobutyl-3,4-Dimethoxybenzoate: 32.5 g of 4'-chlorobutyl-3,4-
dimethoxybenzoate and 19.5 g of sodium iodide (10% excess) were boiled in
150 ml of methyl ethyl ketone for 2.5 hours; after cooling and filtering off the
sodium chloride produced, the reaction was found not to be entirely
completed; boiling was then continued for another two hours; the reaction
mixture was cooled, and the solid filtered off and washed with 2 x 100 ml of
ether.
The filtrate was evaporated to dryness in vacuo and the residue was dissolved
in 300 ml of ether and 100 ml of water; the layers were separated and the
water layer was once again extracted with 100 ml of ether; then the ether
layers were boiled and washed again with a solution of 3.5 g of sodium
thiosulfate in 100 ml of water. The ether layer was dried over sodium sulfate.
Finally the solution was filtered and the ether was evaporated; the residue
was an almost colorless oil, which partially solidified or became more viscous
after being left to stand for some time. Yield: 40 g (=92%), I=34.2%
(calculated 34.9%).
(D) 4'-[N-Ethyl-1''-Methyl-2''-(4'''-Methoxyphenyl)Ethylamino]Butyl-3,4-
Dimethoxybenzoate Hydrochloride: 10.3 g of 4'-iodobutyl-3,4-
dimethoxybenzoate and 11.0 g of N-ethyl-p-methoxyphenylisopropylamine
(obtained by catalytic reduction of an alcoholic solution of an excess quantity
(60%) of p-methoxy-phenyl-acetone, to which was added a 33% (weight-for-weight) aqueous solution of ethylamine, with Pt as a catalyst), were boiled in
200 ml of methyl ethyl ketone for 20 hours, cooled and the iodine ion was
determined; the reaction was found to be complete. Then the methyl ethyl
ketone was evaporated in vacuo and the residue was dissolved in 300 ml of
water and 30 ml of ether; the layers were separated and the water layer was
extracted twice more with 20 ml portions of ether.
Therapeutic Function
Spasmolytic
Properties of Mebeverine hydrochloride
| Melting point: | 105-107 C |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | DMSO : ≥ 155 mg/mL (332.61 mM) |
| form | neat |
| form | Solid |
| pka | pKa 9.07± 0.02(40% EtOH,t =25,) (Uncertain) |
| color | White to off-white |
| CAS DataBase Reference | 2753-45-9(CAS DataBase Reference) |
Safety information for Mebeverine hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for Mebeverine hydrochloride
Mebeverine hydrochloride manufacturer
Gangwal Healthcare Pvt Ltd
SETV ASRV LLP
Festiva Pharma
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran
You may like
-
 2753-45-9 98%View Details
2753-45-9 98%View Details
2753-45-9 -
 MEBEVERINE HYDROCHLORIDE 80.0% SR PELLETS 98%View Details
MEBEVERINE HYDROCHLORIDE 80.0% SR PELLETS 98%View Details
2753-45-9 -
 2753-45-9 MEBEVERINE HCl 95-99%View Details
2753-45-9 MEBEVERINE HCl 95-99%View Details
2753-45-9 -
 2753-45-9 Mebeverine hydrochloride 99%View Details
2753-45-9 Mebeverine hydrochloride 99%View Details
2753-45-9 -
 Mebeverine hydrochloride 98%View Details
Mebeverine hydrochloride 98%View Details
2753-45-9 -
 Mebeverine hydrochloride 99%View Details
Mebeverine hydrochloride 99%View Details -
 Mebeverine hydrochloride 95% CAS 2753-45-9View Details
Mebeverine hydrochloride 95% CAS 2753-45-9View Details
2753-45-9 -
 Mebeverine hydrochloride CAS 2753-45-9View Details
Mebeverine hydrochloride CAS 2753-45-9View Details
2753-45-9
