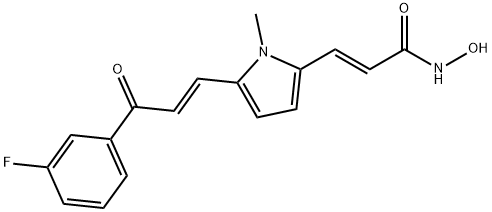
MC1568
Synonym(s):3-[5-(3-(3-Fluorophenyl)-3-oxopropen-1-yl)-1-methyl-1H-pyrrol-2-yl]-N-hydroxy-2-propenamide
- CAS NO.:852475-26-4
- Empirical Formula: C17H15FN2O3
- Molecular Weight: 314.31
- MDL number: MFCD16875423
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-17 16:00:36
What is MC1568?
Description
While class I HDACs are localized predominantly within the nucleus, class II HDACs shuttle into and out of the nucleus in response to intracellular signaling. Class IIa HDACs, which includes HDAC4, 5, 7, and 9, commonly act as corepressors and play diverse roles in cell biology. MC 1568 is a selective inhibitor of class IIa HDACs, with greater than 170-fold selectivity over class I HDACs, including HDAC1. It has been used in cells (1-10 μM) and in mice to elucidate the roles of class IIa HDACs in cell proliferation and differentiation, apoptosis, myogenesis, adipogenesis, and fibrosis.
The Uses of MC1568
A selective class II (IIa) histone deacetylase (HDAC II) inhibitor. It exhibits tissue-selective inhibition between members of class II acetylases in vivo, particularly in skeletal muscle and the heart. It arrests myogenesis through the stabilization of myocyte enhancer factor 2D (MEF2D)-HDAC3/4 complex.
The Uses of MC1568
While class I HDACs are localized predominantly within the nucleus, class II HDACs shuttle into and out of the nucleus in response to intracellular signaling. Class IIa HDACs, which includes HDAC4, 5, 7, and 9, commonly act as corepressors and play diverse roles in cell biology. MC 1568 is a selective inhibitor of class IIa HDACs, with greater than 170-fold selectivity over class I HDACs, including HDAC1. It has been used in cells (1-10 μM) and in mice to elucidate the roles of class IIa HDACs in cell proliferation and differentiation, apoptosis, myogenesis, adipogenesis, and fibrosis.[Cayman Chemical]
What are the applications of Application
MC 1568 is a class II (IIa) HDAC inhibitor selective for HDAC4 and HDAC6
Definition
ChEBI: 3-[4-[3-(3-fluorophenyl)-3-oxoprop-1-enyl]-1-methyl-2-pyrrolyl]-N-hydroxy-2-propenamide is a carbonyl compound.
Biochem/physiol Actions
MC1568 is a selective class II (IIa) histone deacetylas (HDAC II) inhibitor.
storage
+4°C
Properties of MC1568
| Melting point: | 212-215 °C(Solv: acetonitrile (75-05-8); methanol (67-56-1)) |
| Density | 1.21±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: ≥10mg/mL |
| pka | 8.91±0.23(Predicted) |
| form | powder |
| color | orange |
| CAS DataBase Reference | 852475-26-4 |
Safety information for MC1568
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for MC1568
| InChIKey | QRDAPCMJAOQZSU-KQQUZDAGSA-N |
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile Diclofenac Potassium Ornidazole IP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Aceclofenac IP/BP/EP Nimesulide BP SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION Methylcobalamin (vitamin B12) SODIUM METHYL PARABEN SODIUM VALPROATE Racecadotril XANTHAN GUMRelated products of tetrahydrofuran
You may like
-
 MC1568 98.00% CAS 852475-26-4View Details
MC1568 98.00% CAS 852475-26-4View Details
852475-26-4 -
 MC1568 CAS 852475-26-4View Details
MC1568 CAS 852475-26-4View Details
852475-26-4 -
 89796-99-6 Aceclofenac IP/BP/EP 98%View Details
89796-99-6 Aceclofenac IP/BP/EP 98%View Details
89796-99-6 -
 61-68-7 98%View Details
61-68-7 98%View Details
61-68-7 -
 Diclofenac Sodium IP/BP/EP/USP 98%View Details
Diclofenac Sodium IP/BP/EP/USP 98%View Details
15307-79-6 -
 Ornidazole IP 16773-42-5 98%View Details
Ornidazole IP 16773-42-5 98%View Details
16773-42-5 -
 51803-78-2 Nimesulide BP 98%View Details
51803-78-2 Nimesulide BP 98%View Details
51803-78-2 -
 15307-81-0 98%View Details
15307-81-0 98%View Details
15307-81-0