Mandelonitrile
Synonym(s):α-Hydroxyphenylacetonitrile;Benzaldehyde cyanohydrin;Benzaldehyde cyanohydrin, DL-α-Hydroxyphenylacetonitrile;DL-Mandelic acid nitrile;Mandelic acid nitrile
- CAS NO.:532-28-5
- Empirical Formula: C8H7NO
- Molecular Weight: 133.15
- MDL number: MFCD00004487
- EINECS: 208-532-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
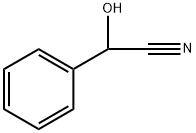
What is Mandelonitrile?
Chemical properties
reddish-brown liquid. D,L-Mandelonitrile [532-28-5], benzaldehyde cyanohydrin, a-cyano-a-hydroxymethylbenzene, C6H5 – CH(OH) – CN, Mr 133.15, mp-10℃, d204 1.1165 – 1.120, is miscible with ethanol and diethyl ether, immiscible with water. Mandelonitrile occurs naturally as the bglycoside of gentiobiose (amygdalin). It decomposes on heating into benzaldehyde and hydrogen cyanide. It is produced from benzaldehyde and alkali cyanide in the presence of mineral acid. Mandelonitrile is used as an intermediate in the production of mandelic acid.
Occurrence
Mandelonitrile, is a yellow, oily liquid, insoluble in water, but soluble in alcohol and diethyl ether. Mandelonitrile is a component of the glycoside amygdalin, a precursor of laetrile found in the leaves and seeds on most Prunus species (plum, peach, apricot, etc). In 1832, mandelonitrile was the first cyanohydrin to be synthesized. It is commercially prepared from benzaldehyde and hydrogen cyanide. Mandelonitrile is used by certain insects (tiger beetles, an African millipede) as a defense fluid. After expelling the fluid an enzyme catalyzes the conversion of mandelonitrile to benzaldehyde and HCN, which is usually fatal to the insect’s enemy.
The Uses of Mandelonitrile
Mandelonitrile has been used to extract mandeloamide by the nitrilase variants.
Definition
ChEBI: Mandelonitrile is a cyanohydrin that is phenylacetonitrile in which one of the methylene hydrogens is replaced by a hydroxy group.
General Description
Reddish-brown to dark red-brown liquid.
Air & Water Reactions
Mandelonitrile is sensitive to moisture. . Insoluble in water.
Reactivity Profile
Nitriles, such as Mandelonitrile, may polymerize in the presence of metals and some metal compounds. They are incompatible with acids; mixing nitriles with strong oxidizing acids can lead to extremely violent reactions. Nitriles are generally incompatible with other oxidizing agents such as peroxides and epoxides. The combination of bases and nitriles can produce hydrogen cyanide. Nitriles are hydrolyzed in both aqueous acid and base to give carboxylic acids (or salts of carboxylic acids). These reactions generate heat. Peroxides convert nitriles to amides. Nitriles can react vigorously with reducing agents. Acetonitrile and propionitrile are soluble in water, but nitriles higher than propionitrile have low aqueous solubility. They are also insoluble in aqueous acids.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Mandelonitrile is combustible.
Safety Profile
Poison by intravenous and subcutaneous routes. Mutation data reported. A severe eye irritant. When heated to decomposition it emits toxic fumes of NOx and CN-. See also NITRILES.
Toxicology
Mandelonitrile is rather toxic with an LD50 value (rat, oral) of 116 mg/kg. This high toxicity may be due to the equilibrium of the cyanohydrin with benzaldehyde and hydrogen cyanide.
Properties of Mandelonitrile
| Melting point: | -10 °C |
| Boiling point: | 170 °C(lit.) |
| Density | 1.117 g/mL at 25 °C(lit.) |
| refractive index | n |
| Flash point: | 207 °F |
| storage temp. | Store below +30°C. |
| solubility | alcohol: freely soluble |
| form | Liquid |
| pka | 10.50±0.20(Predicted) |
| color | Yellow to brown |
| Sensitive | Moisture Sensitive |
| Merck | 14,5719 |
| BRN | 2207122 |
| Dielectric constant | 17.0(23℃) |
| Stability: | Stable, but moisture sensitive. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 532-28-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzeneacetonitrile, «alpha»-hydroxy-(532-28-5) |
| EPA Substance Registry System | Mandelonitrile (532-28-5) |
Safety information for Mandelonitrile
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Mandelonitrile
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran


You may like
-
 532-28-5 Mandelonitrile 98%View Details
532-28-5 Mandelonitrile 98%View Details
532-28-5 -
 Mandelonitrile CAS 532-28-5View Details
Mandelonitrile CAS 532-28-5View Details
532-28-5 -
 Mandelonitrile CAS 532-28-5View Details
Mandelonitrile CAS 532-28-5View Details
532-28-5 -
 DL-Mandelic acid nitrile CAS 532-28-5View Details
DL-Mandelic acid nitrile CAS 532-28-5View Details
532-28-5 -
 17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
