LYSIONOTIN
- CAS NO.:152743-19-6
- Empirical Formula: C18H16O7
- Molecular Weight: 344.317
- MDL number: MFCD08689947
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-18 21:14:07
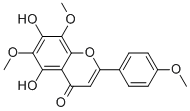
What is LYSIONOTIN?
Description
Chinese stone Chlorophytum is the whole herb of Lysionotus pauciflorus Maxim,
which belongs to Gesneriaceae. Stone Chlorophytum is a small evergreen shrub,
which is born in wet places of rock slope and clings to the stone or a tree. Cutting
stone Chlorophytum in summer and autumn season when leafiness, removing impu rities, and drying. Stone Chlorophytum is more in Jiangsu, Zhejiang, Anhui, Jiangxi,
Hunan, Hubei, Shaanxi, Sichuan, Yunnan, Guizhou, and Guangxi.
Stone Chlorophytum has a long medical history in Yunnan, Guizhou, and south
of Qinling region. The function was described in various previous dynasty medicine
classics. Stone Chlorophytum tastes bitter, is flat, and goes into the lung, liver, and
kidney meridians. It has been recorded in herbal formula that stone Chlorophytum
could eliminate sputum and poison, promote digestion, nourish blood, and cure
rheumatism, emphysema, dizziness, and various kinds of deficiency. In the classifi cation of herbs, it was recorded that stone Chlorophytum could cure hematemesis,
blood vomiting, pain of waist and knee, and contusions. It has been described incommon folk medicinal herbs that stone Chlorophytum could clear the lung to
relieve cough, cool blood, and hemostasis .
Physical properties
Appearance: yellow needle crystal or light yellow powder, odorless, and tasteless. Solubility: slightly soluble in chloroform and in methanol, ethanol, or ethyl acetate, very slightly soluble in ether, insoluble in water, and soluble in 5% sodium carbon ate solution, potassium hydroxide, or sodium hydroxide solution. Melting point: 197–199 °C
History
Stone Chlorophytum is the folk antituberculous herb, the fat-soluble component
separated from which is lysionotin. China’s scientists for the first time found that
lysionotin is the effective component of the antituberculosis in stone
Chlorophytum. Xu Yin et al., who worked in Shanghai Institute of Materia
Medica, Chinese Academy of Sciences, found that lysionotin is 5,7-dihydroxy 4′,6′,8-trimethoxy flavonoids by structure identification, which is the known
compound nevadensin
Farkas et al. used a 14-step synthesis to demonstrate the structure of nevadensin
with complex reaction steps. The synthesis route was simpler, which was designed
by Tang Cheng-fang in Shanghai Institute of Materia Medica, Chinese Academy of
Sciences, and the total yield was ten times higher .
oluble, clinical use of injection caused pain to the patients. In order to overcome the
above shortcomings, methylamine glucoside salt of nevadensin was synthesized for
intramuscular injection. The effect is significant without causing pain. In order to
explore its structure-activity relationship, seven chemical analogues were designed
and synthesized with good activity in antibacterial test of Mycobacterium tuberculo sis, which include 6,8-dichloro-4′-methoxyflavone, 6,7-dimethyl-4′-methoxyflavone,
6,7-dichloro-4′-methoxyflavone, dimethoxy-3′, 4′-dimethoxyflavone,
6,7-dichloro-3′, 4′-dimethoxyflavone, 6-chloro-4′-methoxyflavone, and 4-dichloro 6-(4-methoxybenzoyloxy) acetophenone (Fig. 3)
Indications
It can make expectoration easy, relieve a cough, soften hard lumps, and dispel nodes. It is used clinically for bronchitis, lymph node tuberculosis, chill cold, etc
Definition
ChEBI: Nevadensin is a trimethoxyflavone that is flavone substituted by methoxy groups at positions 6, 8 and 4' and hydroxy groups at positions 5 and 7 respectively. It has a role as a plant metabolite. It is a trimethoxyflavone and a dihydroxyflavone. It is functionally related to a flavone. It is a conjugate acid of a nevadensin-7-olate.
Pharmacology
Anti-Mycobacterium tuberculosis, Anti-inflammatory Effect
Nevadensin 200 μg/ml in vitro has obvious anti-Mycobacterium tuberculosis
effect; in vivo test also has a protective effect. The treatment for lymph node is sig nificant . Nevadensin has significant inhibitory effect on the experimental arthri tis caused by serotonin, formaldehyde, and kaolin. It also has an inhibitory effect on
the cotton ball granuloma. Furthermore, the anti-inflammatory effect does not
depend on the presence of adrenal cortex
Antihypertensive Effect
The main role of nevadensin in the central nervous system causes blood pressure
reduction, which also has an impact on the peripheral nervous system. Lateral
ventricle injection of nevadensin could reduce arterial blood pressure, and the effect
of lowering systolic blood pressure was stronger than that of reducing diastolic
blood pressure. The mechanism might be that the action of nevadensin on
α-adrenergic receptor is stronger than that of the central β-adrenergic receptor.
Scavenging Free Radicals
Phenolic hydroxyl group of nevadensin is the main active group that scavenges
free radicals (OH).
Clinical Use
Nevadensin has been used for the adjuvant therapy of cervical lymph node tuberculosis, tuberculosis, bone tuberculosis, and bronchitis treatment. Adverse reactions were mainly nausea, vomiting, diarrhea and abdominal discomfort, etc
Safety information for LYSIONOTIN
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran





You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4