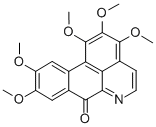
Lysicamine
- CAS NO.:15444-20-9
- Empirical Formula: C18H13NO3
- Molecular Weight: 291.3
- MDL number: MFCD01711485
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-08-28 18:13:45
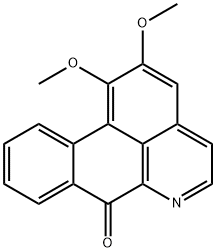
What is Lysicamine?
Description
This oxodibenzoquinoline alkaloid occurs in Lysichiton camtschatcense Schott var. japonicum Makino. Being a fully conjugated molecule, the alkaloid is coloured, yielding yellow needles when crystallized from EtOH. The ultraviolet spectrum in neutral solution (EtOH) has absorption maxima at 235, 270, 307 and 400 mil. In acid solution (EtOH-O.l N/HCl), the absorption maxima occur at 249,276,306 and 453 mil. The structure has been established by synthesis as 1 :2-dimethoxy-7-oxodibenzo[de, gj quinoline.
The Uses of Lysicamine
Lyscamine has shown to have cytotoxicity against various human cancer cells. It can also be enriched using response surface method and adsorption on macroporous resin.
Definition
ChEBI: A natural product found in Annona glabra.
References
Katsui et al., Tetrahedron Lett., 6257 (1966)
Properties of Lysicamine
| Melting point: | 210-1 °c (dec.). |
| Boiling point: | 509.4±45.0 °C(Predicted) |
| Density | 1.323±0.06 g/cm3(Predicted) |
| pka | 2.67±0.20(Predicted) |
Safety information for Lysicamine
Computed Descriptors for Lysicamine
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8