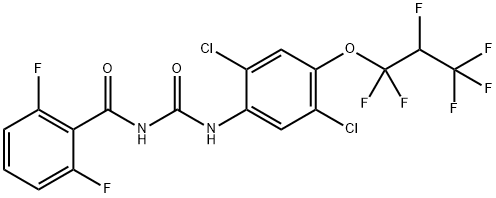
Lufenuron
Synonym(s):1-[2,5-Dichloro-4-(1,1,2,3,3,3-hexafluoropropoxy)phenyl]-3-(2,6-difluorobenzoyl)urea;Lufenuron
- CAS NO.:103055-07-8
- Empirical Formula: C17H8Cl2F8N2O3
- Molecular Weight: 511.15
- MDL number: MFCD00867616
- EINECS: 410-690-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-11-10 14:44:25
What is Lufenuron?
Description
Lufenuron is an insect development inhibitor of the benzoylphenyl urea class. It demonstrates activity against fleas that have fed on treated cats and dogs and become exposed to lufenuron in the host’s blood. Lufenuron also has activity by virtue of its presence in adult flea feces, leading to its ingestion by flea larvae. Both activities result in the production of eggs that are unable to hatch, causing significant reductions in flea larvae populations. The lipophilicity of lufenuron leads to its deposition in adipose tissues of animals from where it is slowly released into the bloodstream. This permits effective blood concentrations to be maintained throughout the recommended oral dosing interval of 1 month.
The Uses of Lufenuron
Lufenuron is used for the control of Lepidoptera and Coleoptera larvae on cotton, maize and vegetables, and of citrus whitefly and rust mites on citrus fruit. It is also used to control fleas on pets and cockroaches in houses.
Definition
ChEBI: Lufenuron is a benzoylurea insecticide, a dichlorobenzene, a N-acylurea, an aromatic ether and an organofluorine compound.
Flammability and Explosibility
Not classified
Pharmacology
Antiparasitic. Lufenuron is a benzoylurea insecticide. This class of insecticides was previously used on fruits to decrease damage by insects. Lufenuron (Program) has been used for prevention of flea infections in dogs and cats because it inhibits chitin synthesis. For this use, it has been given to dogs at a dose of 10 mg/kg every 30 days and to cats at a dose of 30 mg/kg every 30 days. It may also have some inhibition on fungal cell membranes because it inhibits the cell wall of fungi, which contain chitin, and other complex polysaccharides. Because of this property on fungal cell membranes, there has been interest in using lufenuron to treat dermatophytes in small animals. However, proven efficacy has been controversial. Well-controlled studies have not confirmed consistent efficacy for treating dermatophytes in animals.
Veterinary Drugs and Treatments
Lufenuron is approved for use in dogs and cats 6 weeks of age and
older for the control of flea populations. The combination product
of lufenuron and milbemycin (Sentinel?) is indicated
for use
in puppies and dogs 4 weeks and older for prevention and control
flea populations, prevention
of heartworm disease, control of adult
hookworms, and the removal and control of adult roundworms
and whipworms.
Lufeneron showed initial promise as a treatment for fungal infections,
but the early enthusiasm has dampened considerably as
efficacy appears doubtful.
Metabolic pathway
Only limited information is available in the open literature on the metabolism of lufenuron.
Degradation
Lufenuron is less stable at alkaline pH than under acidic conditions. The DT50 at 25 °C is 160 days at pH 5,70 days at pH 7 and 32 days at pH 9 (PM).
Mode of action
Lufenuron has no systemic or translaminar effect. It is persistent with a transovarial effect. It may reduce the egg-Iaying rate or hinder the hatching process of embryos.
Properties of Lufenuron
| Melting point: | 174.1° |
| Density | 1.631±0.06 g/cm3(Predicted) |
| vapor pressure | <0.4 x10 -3 Pa (25 °C) |
| Flash point: | 170 °C |
| storage temp. | 0-6°C |
| solubility | 100mg/L in organic solvents at 20 ℃ |
| form | neat |
| Water Solubility | <0.06 mg l-1(25 °C) |
| pka | 8.49±0.46(Predicted) |
| form | Solid |
| color | Off-white to light yellow |
| JECFA Number | 85 |
| BRN | 8398291 |
| CAS DataBase Reference | 103055-07-8(CAS DataBase Reference) |
| EPA Substance Registry System | Benzamide, N-[[[2,5-dichloro-4-(1,1,2,3,3,3-hexafluoropropoxy)phenyl]amino]carbonyl]-2,6-difluoro- (103055-07-8) |
Safety information for Lufenuron
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P272:Contaminated work clothing should not be allowed out of the workplace. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P333+P313:IF SKIN irritation or rash occurs: Get medical advice/attention. |
Computed Descriptors for Lufenuron
| InChIKey | PWPJGUXAGUPAHP-UHFFFAOYSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Lufenuron 95% CAS 103055-07-8View Details
Lufenuron 95% CAS 103055-07-8View Details
103055-07-8 -
 Lufenuron CAS 103055-07-8View Details
Lufenuron CAS 103055-07-8View Details
103055-07-8 -
 Lufenuron CAS 103055-07-8View Details
Lufenuron CAS 103055-07-8View Details
103055-07-8 -
 Lufenuron CAS 103055-07-8View Details
Lufenuron CAS 103055-07-8View Details
103055-07-8 -
 Lufenuron 50 ec, 50% SCView Details
Lufenuron 50 ec, 50% SCView Details
103055-07-8 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
