Lorcaserin hydrochloride
- CAS NO.:846589-98-8
- Empirical Formula: C11H15Cl2N
- Molecular Weight: 232.15
- MDL number: MFCD09833667
- EINECS: 1592732-453-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-03-07 10:32:25
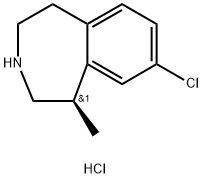
What is Lorcaserin hydrochloride?
Description
In June 2012, the US FDA approved lorcaserin for chronic weight management in adult patients who are characterized as overweight or obese and have at least one comorbid, weight-related condition. Lorcaserin (also known as APD356) is the first 5-HT2C agonist approved for chronic weight management since the withdrawal of the 5-HT2C agonist fenfluramine in 1997 due to rare cases of cardiac valvulopathy. The serotonin receptor 5-HT2C is found primarily in the hypothalamus and regulates appetite and feeding behavior. Safety issues with fenfluramine were associated with poor selectivity for 5-HT2C versus 5HT2A and 5HT2B. Ring constraint afforded by the benzazepine in lorcaserin improved selectivity for 5HT2C by over 2 log units.
Description
Lorcaserin (hydrochloride) (CRM) (Item No. 19247) is a certified reference material categorized as an anorectic. It also decreases oxycodone intake and has positive effects on self-administration and relapse vulnerability in the rat. Lorcaserin is regulated as a Schedule IV compound in the United States. This product is intended for research and forensic applications.
Originator
Pharmaceuticals (United States)
The Uses of Lorcaserin hydrochloride
Lorcaserin hydrochloride, a novel antiobesity drug, is a selective serotonin 5-HT2C receptor agonist, approved by FDA in 2012.
Definition
ChEBI: A hydrochloride obtained by reaction of lorcaserin with one equivalent of hydrochloric acid. Used as an anti-obesity drug.
brand name
Belviq
Side Effects
Lorcaserin hydrochloride (Belviq) is a serotonin 2C receptor agonist that causes weight loss by causing appetite suppression.It appears to have fewer adverse effects than orlistat,although long-term safety data are limited. The recommended dosage is 10mg twice daily. Lorcaserin should be discontinued if patients do not lose 5% of their body weight in 12 weeks. The efficacy of lorcaserin appears similar to that of orlistat (mean difference in weight loss between active and placebo-treated groups approximately 3-4 kg).Common side effects include nausea, headache,dizziness, nasopharyngitis, and fatigue. Lorcaserin may increasethe risk of symptomatic hypoglycemia in patients with type 2 diabetes on oral agents, necessitating a reduction in the dose of diabetes medications. Lorcaserin should not be used in individuals with creatinine clearance <30 mL/minute. It is contraindicated during pregnancy. In addition, lorcaserin should not be used with other serotonergic drugs (e.g., selective serotonin reuptake inhibitors,selective serotonin-norepinephrine reuptake inhibitors, bupropion[Wellbutrin], tricyclic antidepressants,and monamine oxidase inhibitors)because of the theoretical potential for serotonin syndrome.
https://www.accessdata.fda.gov
https://medlineplus.gov
Drug interactions
Belviq (lorcaserin hydrochloride) may interact with antidepressants, including selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), monoamine oxidase inhibitors (MAOIs), triptans, bupropion, dextromethorphan, or St. John's Wort.
Precautions
The precautions of BELVIQ (lorcaserin hydrochloride) tablets:
Serotonin Syndrome or Neuroleptic Malignant Syndrome (NMS)- like Reactions: The safety of coadministration with other serotonergic or antidopaminergic agents has not been established.
Valvular heart disease: If signs or symptoms develop, consider BELVIQ discontinuation and evaluate the patient for possible valvulopathy.
Cognitive Impairment: This may cause disturbances in attention or memory. Caution with the use of hazardous machinery when starting BELVIQ treatment.
Psychiatric Disorders, including euphoria and dissociation: Do not exceed the recommended dose of 10 mg twice daily.
Monitor for depression or suicidal thoughts. Discontinue if symptoms develop.
Use of Antidiabetic Medications: Weight loss may cause hypoglycemia. Monitor blood glucose. BELVIQ has not been studied in patients taking insulin.
Priapism: Patients should seek emergency treatment if an erection lasts >4 hours. Use BELVIQ with caution in patients predisposed to priapism.
Properties of Lorcaserin hydrochloride
| solubility | Soluble in DMSO > 10 mM |
| form | Powder |
| Stability: | Hygroscopic |
Safety information for Lorcaserin hydrochloride
Computed Descriptors for Lorcaserin hydrochloride
Lorcaserin hydrochloride manufacturer
Lupin Ltd
Symed Laboratories Ltd
Archerchem Healthcare Pvt., Ltd. (part of Archerchem Group)
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 846589-98-8 Lorcaserin hydrochloride 99%View Details
846589-98-8 Lorcaserin hydrochloride 99%View Details
846589-98-8 -
 846589-98-8 99%View Details
846589-98-8 99%View Details
846589-98-8 -
 Lorcaserin hydrochloride 846589-98-8 98%View Details
Lorcaserin hydrochloride 846589-98-8 98%View Details
846589-98-8 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1