Lomefloxacin
- CAS NO.:98079-51-7
- Empirical Formula: C17H19F2N3O3
- Molecular Weight: 351.35
- MDL number: MFCD00242673
- EINECS: 619-317-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
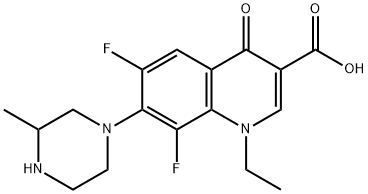
What is Lomefloxacin?
Absorption
Rapid and nearly complete with approximately 95% to 98% of a single oral dose being absorbed.
Toxicity
Adverse reactions include peripheral neuropathy, nervousness, agitation, anxiety, and phototoxic events (rash, itching, burning) due to sunlight exposure.
Description
Lomefloxacin is a once-daily, third-generation quinolone antibiotic useful in the treatment of bacterial infections. The new fluorinated quinolone does not interfere with the metabolism of theophylline; it is efficacious against pathogens resistant to cephalosporins, penicillins and aminoglycosides.
Chemical properties
colorless Needles
Originator
Hokuriku Seiyaku (Japan)
The Uses of Lomefloxacin
Anti bacterial.
Indications
For the treatment of bacterial infections of the respiratory tract (chronic bronchitis) and urinary tract, and as a pre-operative prophylactic to prevent urinary tract infection caused by: S.pneumoniae, H.influenzae, S.aureus, P.aeruginosa, E. cloacae, P. mirabilis, C. civersus, S. asprphyticus, E.coli, and K.pneumoniae.
Background
Lomefloxacin is a fluoroquinolone antibiotic, used to treat bacterial infections including bronchitis and urinary tract infections (UTIs). Additionally, it has been employed for the prophylaxis of UTIs prior to surgery as well.
Definition
ChEBI: A fluoroquinolone antibiotic, used (generally as the hydrochloride salt) to treat bacterial infections including bronchitis and urinary tract infections. It is also used to prevent urinary tract infections prior to surgery.
Manufacturing Process
A mixture of 1.00 g of 1-ethyl-6,7,8-trifluoro-1,4-dihydro-4-oxoquinoline-3-
carboxylic acid, 1.10 g of 2-methylpiperazine and 10 ml of pyridine was
heated for 15 minutes under reflux. The reaction mixture was evaporated and
methanol was added to the residue. The precipitate was filtered and
recrystallized from ethanol to give 0.36 g of the 1-ethyl-6,8-difluoro-1,4-
dihydro-7-(3-methyl-1-piperazinyl)-4-oxoquinoline-3-carboxylic acid as
colorless needles, melting point 239.0-240.5°C.
By the usual manner the hydrochloride was prepared and recrystallized from
water as colorless needles, melting point 290-300°C (decomp.).
brand name
Uniquin
Therapeutic Function
Antibacterial
Pharmaceutical Applications
A difluoropiperazinyl quinolone formulated as the hydrochloride salt for oral administration. The in-vitro activity is very similar to that of norfloxacin . It is active against Enterobacteriaceae and fastidious Gram-negative bacilli, including L. pneumophila. Activity against Campylobacter spp., Ps. aeruginosa, Acinetobacter and Chlamydia spp. is poor. It has reduced activity against staphylococci and poor activity against streptococci, L. monocytogenes, anaerobes and Mycobacterium spp.
A 400 mg oral dose achieves a concentration of 3–5 mg/L after 1–1.5 h. In escalating oral doses of 100, 400 and 800 mg to volunteers, the AUC was essentially proportional to the dosage, the mean plasma concentrations following 100, 400 and 800 mg doses being approximately 1.1, 4.7 and 7.5 mg/L, respectively.
Several metabolites have been described, accounting for <5% of the oral dose. Elimination occurs principally via the kidneys and 50–70% of a dose appears in the urine over 24 h. In patients with impaired renal function given 400 mg orally, the apparent elimination half-life ranged from 8 to 44 h, depending on the degree of renal failure. Non-renal clearance was also impaired, but there was no significant change in other pharmacokinetic parameters. The daily dosage (400 mg) should be reduced to 280 mg when the creatinine clearance falls below 30 mL/min. Hemodialysis has no effect on the plasma concentration. The effect of lomefloxacin on the plasma concentration of theophylline is clinically insignificant and no dosage adjustment is required.
The main adverse event is phototoxicity; other adverse events (mainly diarrhea, abdominal pain, skin reactions, dizziness, headache and insomnia) occur in about 10% of patients.
It is chiefly used in urinary tract infection, but is no longer widely available.
Pharmacokinetics
Lomefloxacin is a fluoroquinolone antibiotic used to treat chronic bronchitis, as well as complicated and uncomplicated urinary tract infections. It is also used as a prophylactic or preventative treatment to prevent urinary tract infections in patients undergoing transrectal or transurethral surgical procedures. Flouroquinolones such as lomefloxacin possess excellent activity against gram-negative aerobic bacteria such as E.coli and Neisseria gonorrhoea as well as gram-positive bacteria including S. pneumoniae and Staphylococcus aureus. They also posses effective activity against shigella, salmonella, campylobacter, gonococcal organisms, and multi drug resistant pseudomonas and enterobacter.
Clinical Use
1-Ethyl-6,8-difluoro-1,4-dihydro-7-(3-methyl-1-piperazinyl)-4-oxo-3-quinolinecarboxylic acid (Maxaquin) is adifluorinated quinolone with a longer elimination half-life(7–8 hours) than other members of its class. It is the onlyquinolone for which once-daily oral dosing suffices. The oralbioavailability of lomefloxacin is estimated to be 95% to98%. Food slows, but does not prevent, its oral absorption.The extent of biotransformation of lomefloxacin is only about5%, and high concentrations of unchanged drug, rangingfrom 60% to 80%, are excreted in the urine. The comparativelylong half-life of lomefloxacin is apparently because ofits excellent tissue distribution and renal reabsorption and not because of plasma protein binding (only ~10%) or enterohepaticrecycling (biliary excretion is estimated to be ~10%).
Lomefloxacin has been approved for two primary indications. First, it is indicated for acute bacterial exacerbations of chronic bronchitis caused by H. influenzae or Moraxella (Branhamella) catarrhalis, but not if Streptococcus pneumoniae is the causative organism. Second, it is used for prophylaxis of infection following transurethral surgery. Lomefloxacin also finds application in the treatment of acute cystitis and chronic urinary tract infections caused by Gram-negative bacilli.
Metabolism
Minimally metabolized although 5 metabolites have been identified in human urine. 65% appears as the parent drug in urine and 9% as the glucuronide metabolite.
Properties of Lomefloxacin
| Melting point: | 239-240 C |
| Boiling point: | 542.7±50.0 °C(Predicted) |
| Density | 1.342±0.06 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Sealed in dry,Store in freezer, under -20°C |
| solubility | DMSO (Slightly), Methanol (Slightly, Heated, Sonicated) |
| pka | -0.25±0.20(Predicted) |
| form | Solid |
| color | Pale Yellow to Light Yellow |
| CAS DataBase Reference | 98079-51-7(CAS DataBase Reference) |
| EPA Substance Registry System | 3-Quinolinecarboxylic acid, 1-ethyl-6,8-difluoro-1,4-dihydro-7-(3-methyl-1-piperazinyl)-4-oxo- (98079-51-7) |
Safety information for Lomefloxacin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H312:Acute toxicity,dermal H332:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Lomefloxacin
Lomefloxacin manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 Lomefloxacin 98% (HPLC) CAS 98079-51-7View Details
Lomefloxacin 98% (HPLC) CAS 98079-51-7View Details
98079-51-7 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1