LINOLENIC ACID ETHYL ESTER
Synonym(s):9,12,15-Octadecatrienoic acid ethyl ester;Linolenic acid ethyl ester
- CAS NO.:1191-41-9
- Empirical Formula: C20H34O2
- Molecular Weight: 306.48
- MDL number: MFCD00009388
- EINECS: 214-734-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
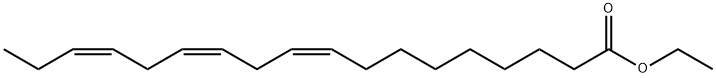
What is LINOLENIC ACID ETHYL ESTER?
Description
α-Linolenic acid ethyl ester is an esterified form of α-linolenic acid (Item Nos. 90210 | 21910). It increases cyclin E levels and the activity of Cdk2/cyclin E, ERK, and JNK in hepatic stellate cells when used at a concentration of 50 μM. α-Linolenic acid ethyl ester (25 μg/ml) inhibits the growth of S. mutans, C. albicans, and P. gingivalis by 98, 72, and 92%, respectively, in vitro. It has been found in biodiesel produced from castor oil using ethanol. α-Linolenic acid ethyl ester has been used as a substrate in lipid peroxidation assays for antioxidant activity.
Chemical properties
clear colorless liquid
The Uses of LINOLENIC ACID ETHYL ESTER
Linolenic Acid Ethyl Ester is the ethyl ester derivative of Linolenic Acid (L467650); an essential fatty acid that occurs mostly as the glyceride in most drying oils. Also a nutrient.
The Uses of LINOLENIC ACID ETHYL ESTER
An exogenous source of α-linolenic acid.
The Uses of LINOLENIC ACID ETHYL ESTER
ethyl linolenate is an emollient reportedly used in indoor tanning preparations. It is not very popular as it may pose rancidity problems. It may also be used as an ingredient to enhance the fragrance of a product.
What are the applications of Application
Linolenic Acid ethyl ester is an exogenous source of α-linolenic acid
Definition
ChEBI: A long-chain fatty acid ethyl ester resulting from the formal condensation of the carboxy group of linolenic acid with the hydroxy group of ethanol.
General Description
Clear colorless liquid.
Air & Water Reactions
Slowly oxidized in air
Reactivity Profile
LINOLENIC ACID ETHYL ESTER is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. LINOLENIC ACID ETHYL ESTER is sensitive to light. LINOLENIC ACID ETHYL ESTER is incompatible with strong oxidizers, strong acids and strong bases.
Fire Hazard
LINOLENIC ACID ETHYL ESTER is probably combustible.
Biochem/physiol Actions
Ethyl linolenate may be used as a reference molecule in systems developed to measure fatty acid esters derived from tissues, membranes and lipids, especially associated with alcohol abuse. Ethyl linolenate is being studied as a possible skin whitening agent with anti-melanogenesis activity.
Properties of LINOLENIC ACID ETHYL ESTER
| Boiling point: | 166-168 °C/1 mmHg (lit.) |
| Density | 0.892 g/mL at 25 °C (lit.) |
| refractive index | n |
| Flash point: | 113 °C |
| storage temp. | -20°C |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly) |
| form | Oil |
| color | Colourless |
| BRN | 1728340 |
| Stability: | Light Sensitive |
| CAS DataBase Reference | 1191-41-9(CAS DataBase Reference) |
| EPA Substance Registry System | 9,12,15-Octadecatrienoic acid, ethyl ester, (9Z,12Z,15Z)- (1191-41-9) |
Safety information for LINOLENIC ACID ETHYL ESTER
Computed Descriptors for LINOLENIC ACID ETHYL ESTER
| InChIKey | JYYFMIOPGOFNPK-AGRJPVHOSA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Ethyl linolenate CAS 1191-41-9View Details
Ethyl linolenate CAS 1191-41-9View Details
1191-41-9 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1