165800-03-3
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Color/Form | White crystals from ethyl acetate and hexanes |
| Melting Point | 154.5 |
| Solubility | 3 mg/mL |
| LogP | 0.9 |
| Optical Rotation | Specific optical rotation at 20 °C for D (sodium) line = -9 deg (c = 0.919 in chloroform) |
| Dissociation Constants | 1.8 |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H372:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. |
COMPUTED DESCRIPTORS
| Molecular Weight | 337.35 g/mol |
|---|---|
| XLogP3 | 0.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 4 |
| Exact Mass | 337.14378429 g/mol |
| Monoisotopic Mass | 337.14378429 g/mol |
| Topological Polar Surface Area | 71.1 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 472 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
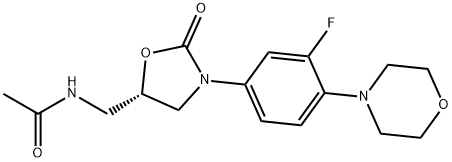
Linezolid is an organofluorine compound that consists of 1,3-oxazolidin-2-one bearing an N-3-fluoro-4-(morpholin-4-yl)phenyl group as well as an acetamidomethyl group at position 5. A synthetic antibacterial agent that inhibits bacterial protein synthesis by binding to a site on 23S ribosomal RNA of the 50S subunit and prevents further formation of a functional 70S initiation complex. It is used for treating serious resistant Gram-positive bacterial infections, such as methicillin-resistant Staphylococcus aureus, penicillin-resistant Streptococcus, and vancomycin-resistant Enteroccocus, which blocks protein synthesis by binding to the 50S portion of the ribosome. Linezolid can be administered either intravenously or orally owing to its absolute bioavailability of close to 100%.
Pharmacokinetics
Linezolid is well absorbed, with a bioavailability of approximately 100% in healthy volunteers. This characteristic is a significant benefit, allowing this agent to be used early intravenously, then switched to oral, or even to commence infection treatment with oral therapy. After oral doses of 600 mg, steady-state peak serum concentrations (Cmax) are 15–27 mg/L and are reached 0.5–2 h after administration. The level of plasma protein binding is 31%, and the volume of distribution approximates the total body water content of 40–50 L. Plasma elimination half-life is 3.4–7.4 h. Linezolid is metabolized into two inactive metabolites, an aminoethoxyacetic acid (metabolite A) and a hydroxyethyl glycine (metabolite B). The clearance rate (±SD) is 80 ± 29 mL/min and by non-renal (65%) and renal mechanisms. Renal tubular reabsorption may occur. A proportion of the dose is excreted unchanged in the urine.
