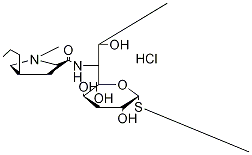
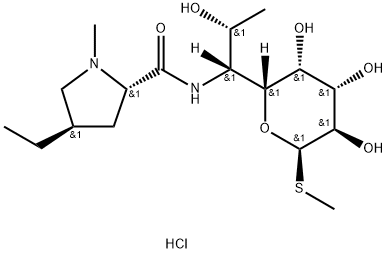
Lincomycin hydrochloride
Synonym(s):Lincocin hydrochloride;Lincomycin hydrochloride monohydrate;Methyl 6,8-dideoxy-6-(1-methyl-4-propyl-2-pyrrolidinecarboxamido)-1-thio-D -erythro-α-D -galactooctopyranoside hydrochloride
- CAS NO.:859-18-7
- Empirical Formula: C18H35ClN2O6S
- Molecular Weight: 443
- MDL number: MFCD00083647
- EINECS: 212-726-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
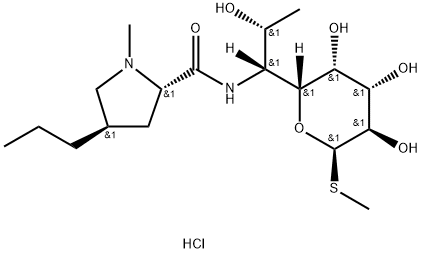
What is Lincomycin hydrochloride?
Chemical properties
Crystalline
Originator
Lincocin,Upjohn,UK,1964
The Uses of Lincomycin hydrochloride
Lincomycin hydrochloride is a salt of lincomycin formed at the basic N-methylproline. The hydrochloride salt is the preferred formulation for pharmaceutical use. Lincomycin hydrochloride is a broad spectrum antibiotic with activity against anaerobic bacteria and protozoans. Lincomycin acts by binding to the 23S ribosomal subunit, blocking protein synthesis. Lincomycin has been extensively studied with over 7,000 literature citations.
The Uses of Lincomycin hydrochloride
An antibiotic produced by Streptomyces lincolnensis. Lincomycin is a lincosamide antibiotic that forms cross-links within the peptidyl transferase loop region of the 23S rRNA. Inhibits bacterial protein synthesis. Antibacterial.
The Uses of Lincomycin hydrochloride
Antibacterial;Ribosomal protein synthesis inhibitor
What are the applications of Application
Lincomycin (U-10149A) is a supressor of protein synthesis in bacteria
Manufacturing Process
As described in US Patent 3,086,912, the process comprises cultivating Streptomyces lincolnensis var. lincolnensis in an aqueous nutrient medium containing a source of assimilable carbohydrate and assimilable nitrogen under aerobic conditions until substantial activity is imparted to the medium by production of lincolnensin and isolating the lincolnensin so produced.
brand name
Lincocin (Pharmacia & Upjohn).
Therapeutic Function
Antibacterial
General Description
Chemical structure: macrolide
Biochem/physiol Actions
Mode of action: Lincomycin inhibits bacterial protein synthesis by forming cross-links within the peptidyl transferase loop region of the 23S rRNA.? Antimicrobial spectrum: Lincomycin hydrochloride is effective against gram-positive bacteria.
Clinical Use
Lincomycin is used for the treatment of infections causedby Gram-positive organisms, notably staphylococci, β-hemolyticstreptococci, and pneumococci. It is absorbed moderatelywell orally and distributed widely in the tissues.Effective concentrations are achieved in bone for the treatmentof staphylococcal osteomyelitis but not in the cerebrospinalfluid for the treatment of meningitis. At one time,lincomycin was considered a nontoxic compound, with alow incidence of allergy (rash) and occasional GI complaints(nausea, vomiting, and diarrhea) as the only adverseeffects. Recent reports of severe diarrhea and the developmentof pseudomembranous colitis in patients treated withlincomycin (or clindamycin), however, have necessitatedreappraisal of the role of these antibiotics in therapy. In anyevent, clindamycin is superior to lincomycin for thetreatment of most infections for which these antibiotics areindicated.
Lincomycin hydrochloride occurs as the monohydrate, awhite, crystalline solid that is stable in the dry state. It isreadily soluble in water and alcohol, and its aqueous solutionsare stable at room temperature. It is degraded slowlyin acid solutions but is absorbed well from the GI tract.Lincomycin diffuses well into peritoneal and pleural fluidsand into bone. It is excreted in the urine and the bile. It isavailable in capsule form for oral administration and inampules and vials for parenteral administration.
Properties of Lincomycin hydrochloride
| Melting point: | 156-158C |
| storage temp. | 2-8°C |
| solubility | H2O: 50 mg/mL, clear, colorless |
| form | solid |
| color | white to white with yellow cast |
| Water Solubility | Soluble in water. |
| BRN | 4171650 |
| Stability: | Hygroscopic |
| EPA Substance Registry System | D-erythro-.alpha.-D-galacto-Octopyranoside, methyl 6,8-dideoxy-6-[[[(2S,4R)-1-methyl-4-propyl-2-pyrrolidinyl]carbonyl]amino]-1-thio-, hydrochloride (1:1) (859-18-7) |
Safety information for Lincomycin hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Lincomycin hydrochloride
| InChIKey | POUMFISTNHIPTI-BOMBIWCESA-N |
| SMILES | [C@]([H])([C@]1([H])[C@@H]([C@H](O)[C@@H](O)[C@@H](SC)O1)O)([C@H](O)C)NC([C@H]1N(C[C@H](CCC)C1)C)=O.Cl |&1:0,2,4,5,7,9,14,19,22,r| |
New Products
3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Iodophenylacetic acid Cyclohexane, (2-propynyloxy)- (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine Pivalic anhydride,98% Phenylmethanesulfonyl fluoride, 98% Glyoxylic acid solution, 50% in water tert-Butyl glycinate,97% 4-Ethoxybenzoic acid, 99% Sodium 1-octanesulfonate monohydrate 7-Ethyl Tryptophol 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl N, N-Carbonyldiimidazole (CDI) 5-Cyanophthalide 10-Methoxy-5H-dibenz[b,f]azepine 3-Methoxybenzonitrile Dibenzoyl Peroxide 4-Methoxybenzonitrile Titanium Dioxide Chloral PentachlorobenzonitrileRelated products of tetrahydrofuran

![2-CHLORO-ALPHA-[2-DIMETHYLAMINOETHYL]BENZHYDROL HYDROCHLORIDE](https://img.chemicalbook.in/CAS/GIF/511-13-7.gif)

You may like
-
 Lincomycin HCl 98.00% CAS 859-18-7View Details
Lincomycin HCl 98.00% CAS 859-18-7View Details
859-18-7 -
 Lincomycin hydrochloride CAS 859-18-7View Details
Lincomycin hydrochloride CAS 859-18-7View Details
859-18-7 -
 Lincomycin hydrochloride CAS 859-18-7View Details
Lincomycin hydrochloride CAS 859-18-7View Details
859-18-7 -
 Lincomycin hydrochloride CAS 859-18-7View Details
Lincomycin hydrochloride CAS 859-18-7View Details
859-18-7 -
 Lincomycin hydrochloride CAS 859-18-7View Details
Lincomycin hydrochloride CAS 859-18-7View Details
859-18-7 -
 Avibactam Sodium SaltView Details
Avibactam Sodium SaltView Details
1192491-61-4 -
 Avibactam intermediateView Details
Avibactam intermediateView Details
1171080-45-7 -
 Relibatan intermediateView Details
Relibatan intermediateView Details
1416134-63-8
