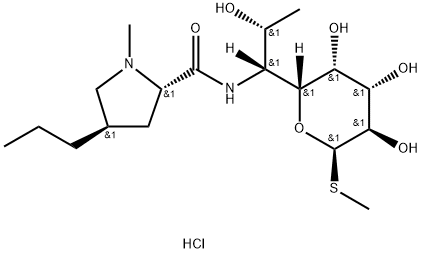
Clindamycin hydrochloride
Synonym(s):(7S)-7-Chloro-7-deoxylincomycin hydrochloride monohydrate;(7S)-7-Chloro-7-deoxylincomycin hydrochloride;Cleocin;Clindamycin hydrochloride monohydrate
- CAS NO.:21462-39-5
- Empirical Formula: C18H34Cl2N2O5S
- Molecular Weight: 461.44
- MDL number: MFCD07793327
- EINECS: 244-398-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-15 14:23:52
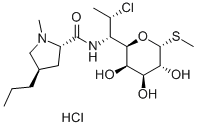
What is Clindamycin hydrochloride?
Chemical properties
White Solid
Originator
Dalacin-C,Diethelm,Switz.,1968
The Uses of Clindamycin hydrochloride
Clindamycin hydrochloride is a salt of clindamycin, a semi-synthetic lincosamide. The hydrochloride salt forms at the basic N-ethylproline moiety and is the preferred pharmaceutical formulation. Like other members of the lincosamide family, clindamycin is a broad spectrum antibiotic with activity against anaerobic bacteria and protozoans. Clindamycin hydrochloride acts by binding to the 23S ribosomal subunit, blocking protein synthesis. Clindamycin hydrochloride has been extensively studied with over 8,000 literature citations.
The Uses of Clindamycin hydrochloride
antibacterial, inhibits protein synthesis
The Uses of Clindamycin hydrochloride
Labelled Clindamycin. Semi-synthetic antibiotic prepared from Lincomycin;Labeled Clindamycin, intended for use as an internal standard for the quantification of Clindamycin by GC- or LC-mass spectrometry.
What are the applications of Application
Clindamycin Hydrochloride is a suppressor of protein synthesis in bacteria
Definition
ChEBI: Clindamycin hydrochloride is a S-glycosyl compound.
Manufacturing Process
The following procedure is described in US Patent 3,475,407. A solution of 50
g of lincomycin hydrochloride, 120 g of triphenylphosphine, and 500 ml of
acetonitrile in a 3 liter flask equipped with a stirrer was cooled in an ice bath
and 500 ml of carbon tetrachloride was added in one portion. The reaction
mixture was then stirred for 18 hours without addition of ice to the cooling
bath. The reaction was evaporated to dryness under vacuum on a 50° to 60°C
water bath, yielding a clear, pale yellow viscous oil. An equal volume of water
was added and the mixture shaken until all of the oil was dissolved. The
resulting suspension of white solid (Ph3PO) was filtered through a sintered
glass mat and discarded. The filtrate was adjusted to pH 11 by addition of 6 N
aqueous sodium hydroxide. A solid precipitated.
The resulting slurry was extracted with four 300 ml portions of chloroform.
The aqueous phase was discarded. The combined chloroform extract was
washed once with 100 ml of saturated aqueous sodium chloride solution and
the sodium chloride phase was discarded. The chloroform phase was
evaporated to dryness under vacuum on a 50° to 60°C water bath and an
equal volume of methanol was added to the residue and the resulting solution
heated at reflux for 1 hour. The methanol solution was evaporated to dryness
under vacuum on a 50° to 60°C water bath. The residue was a clear pale
yellow viscous oil. An equal volume of water and 10 ml of 37% aqueous HCl
was added and the resultant was shaken until the oil dissolved and a white
solid (more Ph3PO) remained in suspension. The suspension was filtered
through a sintered glass mat at pH 1 to 2 and the solid discarded.
The filtrate was extracted twice with 100 ml of carbon tetrachloride. The
carbon tetrachloride phase was discarded. The aqueous phase was adjusted to
pH 11 by addition of 6 N aqueous sodium hydroxide and extracted four times
with 300 ml portions of chloroform. The combined chloroform extract was
washed three times with 100 ml of saturated aqueous sodium chloride
solution and the sodium chloride phase was discarded. The chloroform extract
was dried over anhydrous magnesium sulfate, filtered and the filtrate
evaporated to dryness under vacuum on a 50° to 60 °C water bath. The
residue was a clear, colorless glass weighing 45 g analyzing about 95% 7(S)-
chloro-7-deoxylincomycin. To the crude product there was added 100 ml of
ethanol with warming until a clear solution was obtained. Then 150 ml ethyl
acetate was added and the resultant filtered through a glass mat and the
filtrate adjusted to pH 1 by the addition of saturated ethanolic HCl.
Crystallization soon occurred. The resultant was allowed to stand at 0°C for
18 hours and then filtered through a sintered glass mat. The solid was dried
under vacuum at 60°C for 18 hours yielding 35 g, a 67% yield of 7(S)-chloro-
7-deoxylincomycin hydrochloride as an ethanol solvate.
brand name
Cleocin (Pharmacia & Upjohn).
Therapeutic Function
Antibacterial
General Description
Chemical structure: macrolide
Contact allergens
This lincosanide antibiotic is used in topical form for acne, or systemically has been responsible for exanthematous rashes and acute generalized exanthematous pustulosis.
Biochem/physiol Actions
Clindamycin hydrochloride is highly effective against anaerobic species.
Clinical Use
Clindamycin is recommended for the treatment of a widevariety of upper respiratory, skin, and tissue infections causedby susceptible bacteria. Its activity against streptococci,staphylococci, and pneumococci is indisputably high, and it isone of the most potent agents available against somenon–spore-forming anaerobic bacteria, the Bacteroides spp.in particular. An increasing number of reports of clindamycin-associated GI toxicity, which range in severity fromdiarrhea to an occasionally serious pseudomembranous colitis,have, however, caused some clinical experts to call for areappraisal of the role of this antibiotic in therapy.Clindamycin- (or lincomycin)-associated colitis may be particularlydangerous in elderly or debilitated patients and hascaused deaths in such individuals. The colitis, which is usuallyreversible when the drug is discontinued, is now believedto result from an overgrowth of a clindamycin-resistant strainof the anaerobic intestinal bacterium Clostridium difficile.229The intestinal lining is damaged by a glycoprotein endotoxinreleased by lysis of this organism.
The glycopeptide antibiotic vancomycin has been effectivein the treatment of clindamycin-induced pseudomembranouscolitis and in the control of the experimentally induced bacterial condition in animals. Clindamycin shouldbe reserved for staphylococcal tissue infections, such as cellulitisand osteomyelitis, in penicillin-allergic patients andfor severe anaerobic infections outside the central nervoussystem. Ordinarily, it should not be used to treat upper respiratorytract infections caused by bacteria sensitive to othersafer antibiotics or in prophylaxis.
Veterinary Drugs and Treatments
Clindamycin products are approved for use in dogs and cats. The labeled indications for dogs include wounds, abscesses and osteomyelitis caused by Staphylococcus aureus. Because clindamycin has excellent activity against most pathogenic anaerobic organisms, it is also used extensively for those infections. Clindamycin is used for a variety of protozoal infections, including toxoplasmosis. For further information, refer to the Dosage or Pharmacology sections.
Storage
-20°C
Properties of Clindamycin hydrochloride
| Melting point: | 141°C |
| storage temp. | 2-8°C |
| solubility | H2O: 50 mg/mL, clear, colorless |
| form | neat |
| form | Solid |
| color | White to Off-White |
| BRN | 4070786 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 21462-39-5(CAS DataBase Reference) |
Safety information for Clindamycin hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H362:Reproductive toxicity, effects on or via lactation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P263:Avoid contact during pregnancy/while nursing. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Clindamycin hydrochloride
| InChIKey | AUODDLQVRAJAJM-XJQDNNTCSA-N |
| SMILES | [C@@H]1([C@@H]([C@H](C)Cl)NC(=O)[C@@H]2C[C@@H](CCC)CN2C)[C@H](O)[C@H](O)[C@H]([C@@H](SC)O1)O.Cl |&1:0,1,2,8,10,17,19,21,22,r| |
Clindamycin hydrochloride manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
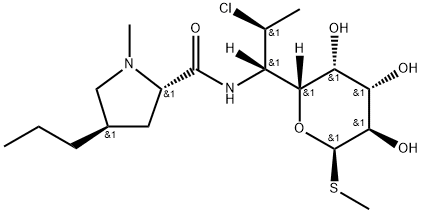
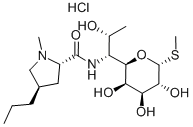

You may like
-
 21462-39-5 Clindamycin Hydrochloride 98%View Details
21462-39-5 Clindamycin Hydrochloride 98%View Details
21462-39-5 -
 Clindamycin hydrochloride 98%View Details
Clindamycin hydrochloride 98%View Details
21462-39-5 -
 21462-39-5 98%View Details
21462-39-5 98%View Details
21462-39-5 -
 Clindamycin Phosphate EP Impurity E 21462-39-5 95.65View Details
Clindamycin Phosphate EP Impurity E 21462-39-5 95.65View Details
21462-39-5 -
 Clindamycin hydrochloride, 97% CAS 21462-39-5View Details
Clindamycin hydrochloride, 97% CAS 21462-39-5View Details
21462-39-5 -
 Clindamycin hydrochloride >98% (HPLC) CAS 21462-39-5View Details
Clindamycin hydrochloride >98% (HPLC) CAS 21462-39-5View Details
21462-39-5 -
 Clindamycin hydrochloride CAS 21462-39-5View Details
Clindamycin hydrochloride CAS 21462-39-5View Details
21462-39-5 -
 Clindamycin hydrochloride CAS 21462-39-5View Details
Clindamycin hydrochloride CAS 21462-39-5View Details
21462-39-5
