Levobunolol hydrochloride
- CAS NO.:27912-14-7
- Empirical Formula: C17H26ClNO3
- Molecular Weight: 327.85
- MDL number: MFCD03425587
- EINECS: 248-725-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-09-04 15:51:00
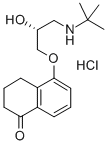
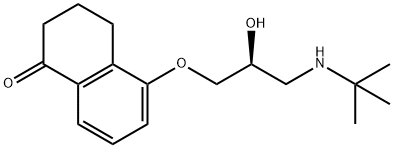
What is Levobunolol hydrochloride?
Description
Levobunolol hydrochloride is a non-selective β-adrenergic blocker useful in the treatment of glaucoma. Worldwide, it represents the sixth B-blocker to be launched for this indication.
Originator
Warner Lambert (USA)
The Uses of Levobunolol hydrochloride
Anti-adrenergic (β-receptor).
The Uses of Levobunolol hydrochloride
A non-selective ?-adrenoceptor antagonist used as an anticonvulsant.
What are the applications of Application
Levobunolol, Hydrochloride is a β-adrenoceptor antagonist
Definition
ChEBI: A hydrochloride obtained by combining equimolar amounts of levobunolol and hydrochloric acid. A non-selective beta-adrenergic antagonist used for treatment of glaucoma.
Manufacturing Process
9.62 g (59 mmoles) 5-hydroxy-3,4-dihydro-1(2H)-naphthalenone, 67 ml
toluene, 0.36 g (1.1 mmoles) tetra-n-butylammonium bromide, 4.51 g (68
mmoles) 85% potassium hydroxide and 20 ml (254 mmoles) (R)-(-)-
epichlorhydrine were placed in an appropriate flask fitted with efficient
mechanical stirring, and the mixture was heated under reflux for two hours.
The mixture was allowed to cool to 30°C, 50 ml toluene and 50 ml water were
added and the mixture was vigorously stirred. The organic phase was
removed and the aqueous phase extracted with 25 ml toluene. The combined
organic phases were concentrated at reduced pressure, 31 ml (300 mmoles)
tert-butylamine, 45 ml ethanol and 3 ml deionized water were added, and the
solution was heated under reflux for one hour. The mixture was allowed to
cool to 40°C and the volatile products were distilled at reduced pressure.
Toluene (9 ml) was added to the residue and volatiles were distilled at
reduced pressure. (S)-5-(2,3-Epoxypropoxy)-3,4-dihydro-1(2H)-
naphthalenone with an optical purity greater than 95% was obtained. Toluene
(75 ml) was added to the product, and then, 10 ml of 35% (w/v) hydrochloric
acid and 110 ml water, and the mixture was stirred for fifteen minutes. The
organic phase was decanted and the aqueous one was extracted with 50 ml
toluene. The aqueous phase was basified by addition of a solution of 5.1 g
sodium hydroxide in 150 ml water and extracted twice with toluene (100 and
50 ml, respectively). The combined organic extracts were dried with
anhydrous sodium sulfate, decolorized with active charcoal and filtered.
To the above toluenic solution containing levobunolol as free base, 16 ml
ethanol and the stoichiometric amount of hydrogen chloride were added. The
stirred mixture was cooled below 10°C and kept at this temperature for one
hour. The precipitated solid was filtered, washed with toluene, recrystallized
twice from 43 ml ethanol and dried to give 10.0 g (51% yield) of (-)-3,4-
dihydro-5-(3-(tert-butylamino)-2-hydroxypropoxy)-1(2H)-naphthalenone
hydrochloride (levobunolol hydrochloride) having a rotary power at 25°C
below -19°.
brand name
Akbeta(Akorn); Betagan(Allergan);VISTAGEN LIQUIFILM.
Therapeutic Function
Beta-adrenergic blocker
Veterinary Drugs and Treatments
Levobunolol HCl is a beta1- and beta2-blocking agent similar to timolol and metipranolol above but without the potential for myocardial depression or airway constriction noted rarely in veterinary medicine and occasionally in human patients. Levobunolol is used in humans with glaucoma responsive to beta adrenergic blocking agents but who suffer cardiac and respiratory side effects associated with timolol. Levobunolol HCl and then carteolol HCl would be suitable Beta blocking agents for feline patients with glaucoma and asthma, although carbonic anhydrase inhibitors should be used in such cases prior to adding a Beta blocking agent.
Properties of Levobunolol hydrochloride
| Melting point: | 209-211° |
| alpha | 24589 -19.6±0.7° (c = 2.90 in methanol) |
| storage temp. | Amber Vial, Refrigerator, Under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly), Water (Sparingly) |
| form | Solid |
| color | White to Off-White |
| Stability: | Light Sensitive |
Safety information for Levobunolol hydrochloride
Computed Descriptors for Levobunolol hydrochloride
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1