102767-28-2
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
PRODUCT INTRODUCTION
Description
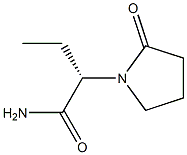
Levetiracetam is an (S)-enantiomer of the ethyl analog of piracetam, in the class of nootropic drugs that are considered to be “pharmacologically safe.” Levetiracetam is approved for partial seizures. The parenteral form is approved as an alternative for the treatment of partial seizures if the oral form is not feasible. It is structurally unrelated to any other antiepileptic class and has a novel mechanism of action. Although the precise mechanism is unknown, it has been shown to bind to synaptic vesicle protein SV2A in animal models. This protein has been related to the modulation of synaptic vesicle exocytosis and neurotransmitter release. Animal models show that the affinity for SV2A is associated with protection against seizures, making it an essential target for new AEDs. In vitro studies demonstrated oppositional activity to negative modulators of gamma-aminobutyric acid (GABA)-gated currents despite a lack of binding affinity to GABA receptors.
