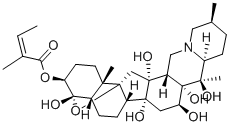
lasiocarpine
- CAS NO.:303-34-4
- Empirical Formula: C21H33NO7
- Molecular Weight: 411.49
- MDL number: MFCD31631257
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
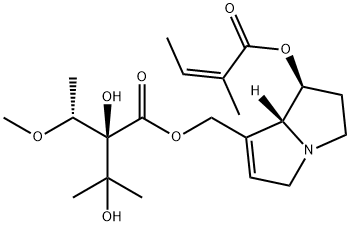
What is lasiocarpine?
Description
A hepatotoxic alkaloid found in Heliotropiurn lasiocarpurn, the base forms colourless leaflets from petroleum ether and has [α]20D - 4° (EtOH). It is readily soluble in C6H6 or EtOH but only slightly so in H20. On alkaline hydrolysis it furnishes angelic acid and heliotridine, C8H1302N, m.p. 116.5-118°C, forming a hydrochloride, m.p. 122-4°C and the benzoyl derivative hydrochloride, m.p. 180°C. On catalytic hydrogenation it yields a base, C13H230 2N, b.p. 123-5°Cj 8 mm; [α]D + 3.8° (EtOH), yielding a crystalline picrate, m.p. 157 _9°C, and lasiocarpic acid, C8H160 S, m.p. 95-7°C; [α]D + 10.6° (EtOH). The structure is therefore angelyllasiocarpylheliotridine, both hydroxyls in heliotridine being es terifie d
Chemical properties
colourless to beige crystalline solid
The Uses of lasiocarpine
Lasiocarpine is a hepatotoxic and carcinogenic food contaminant.
General Description
Colorless plates or beige crystalline solid.
Air & Water Reactions
Decomposes slowly on standing in air at room temperature. Insoluble in water.
Reactivity Profile
lasiocarpine is readily hydrolyzed by alkali. Reacts readily with oxidizing agents. Reacts slowly with atmospheric oxygen .
Health Hazard
ACUTE/CHRONIC HAZARDS: When heated to decomposition lasiocarpine emits toxic fumes of nitrogen oxides.
Fire Hazard
Flash point data for lasiocarpine are not available; however, lasiocarpine is probably combustible.
References
Men'shikov., Ber., 65,974 (1932)
Properties of lasiocarpine
| Melting point: | 96.5-97.0℃ |
| Boiling point: | 531.2°C (rough estimate) |
| alpha | D -4° (10% alc) |
| Density | 1.21±0.1 g/cm3 (20 ºC 760 Torr) |
| refractive index | 1.5614 (estimate) |
| pka | 11.99±0.29(Predicted) |
| Water Solubility | 6.754g/L(temperature not stated) |
| Stability: | Stable, but is readily hydrolyzed in basic solution and readily oxidized by oxidizing agents. Reacts slowly with atmospheric oxygen. |
| IARC | 2B (Vol. 10, Sup 7) 1987 |
| EPA Substance Registry System | Lasiocarpine (303-34-4) |
Safety information for lasiocarpine
Computed Descriptors for lasiocarpine
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![2-[2-(Methylamino)benzoyl]-3,4-dihydro-β-carboline-1(2H)-one](https://img.chemicalbook.in/CAS/20150408/GIF/526-43-2.gif)

You may like
-
 Lasiocarpine CAS 303-34-4View Details
Lasiocarpine CAS 303-34-4View Details
303-34-4 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1