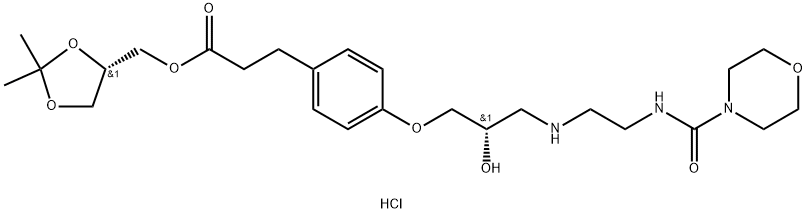
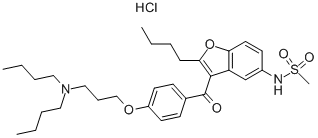
Landiolol hydrochloride
Synonym(s):4-[(2S)-2-Hydroxy-3-[[2-[(4-morpholinylcarbonyl)amino]ethyl]amino]propoxy]-benzenepropanoic acid [(4S)-2,2-dimethyl-1,3-dioxolan-4-yl]methyl ester hydrochloride;4-[2-Hydroxy-3-[[2-[(4-morpholinylcarbonyl)amino]ethyl]amino]propoxy]-benzenepropanoic acid [S-(R*,R*)]-(2,2-dimethyl-1,3-dioxolan-4-yl)methyl ester hydrochloride;ONO 1101;ONO-1101
- CAS NO.:144481-98-1
- Empirical Formula: C25H40ClN3O8
- Molecular Weight: 546.06
- MDL number: MFCD01937430
- EINECS: 1308068-626-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-01 18:09:03
What is Landiolol hydrochloride?
Description
Landiolol hydrochloride (14) was launched in Japan by Ono for the treatment of intraoperative tachyarrhythmia. It improves tachyarrhythmia by selectively blocking β1 receptors located mainly in the heart and by inhibiting the action of catecholamine.
The Uses of Landiolol hydrochloride
Landiolol is an ultra-short acting beta blocker that is used to treat patients with cardiac arrhythmias and is also used to treat tachycardia during anasthesia.
Definition
ChEBI: Landiolol hydrochloride is a member of morpholines.
Pharmacokinetics
Landiolol hydrochloride has a β1/β2 ratio of 255, and, compared to propranolol, its β1-selectivity is 74–380 times greater while it has a 33–263 times greater β1 affinity than esmolol. This high β1-selectivity produces a more potent negative chronotropic effect and a less potent hypotensive effect. Work in rabbit animal models revealed a more rapid HR decrease with landiolol than with esmolol. In contrast, the decrease in mean arterial blood pressure (MAP) seen with esmolol was not observed with landiolol. Similar results were obtained in isolated rabbit and guinea pig hearts. Landiolol metabolism is mainly in the liver (approximately half) and plasma and is predominantly catalyzed by liver carboxylesterase and plasma pseudocholinesterase. In hepatically impaired patients, maximum concentration (Cmax) and area under the concentration (AUC)?time curve values were 42% and 44% higher, respectively. However, half-life did not differ compared with healthy patients, and no drug-related adverse events were observed[1].
Clinical Use
Landiolol hydrochloride, a highly cardio-selective beta-1 blocker with an ultra-short-acting half-life of 4 minutes, is widely used in Japan under the brand name Onoact®. β-blockers is useful in preventing refractory and urgent fatal arrhythmia and tachycardia in chronic phase.Recently, European Union health authorities have approved usage under the name Rapibloc®. Landiolol was originally approved by Japan for treatment of intraoperative tachyarrhythmias and was later approved for tachycardia atrial fibrillation (AF) and atrial flutter (AFL) during left ventricular (LV) dysfunction in November 2013. More recently, landiolol expanded its utility into usage for fatal arrhythmia requiring emergency treatment in 20196 and is fast becoming a reliable therapeutic choice for management of arrhythmia in acute phase[1].
Synthesis
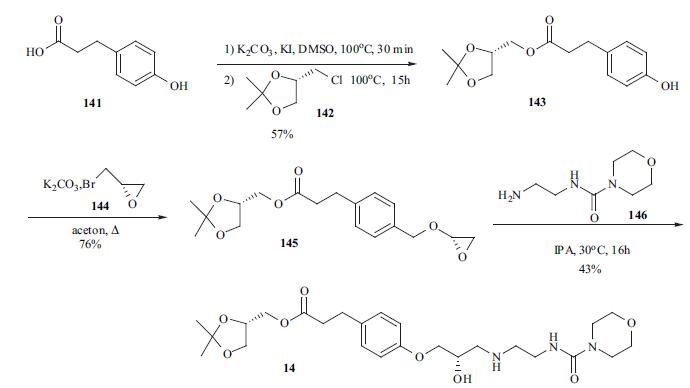
The synthesis of landiolol appeared in an earlier patent in 1990. Esterification of 3- (4-hydroxyphenyl)propionic acid (141) with 2,2-dimethyl- 1,3-dioxolan-4-ylmethyl chloride (142) in DMSO gave desired ester 143 in 57% yield. Treatment of phenol 143 with bromo epoxide 144 in the present of K2CO3 afforded ether 145 in 76% yield. Epoxide 145 was then reacted with free amine 146 via a neucleophilic ring opening process to provide landiolol (14).
References
[1] Yujiro Matsuishi. “Evaluating the Therapeutic Efficacy and Safety of Landiolol Hydrochloride for Management of Arrhythmia in Critical Settings: Review of the Literature.” Vascular Health and Risk Management 16 (2020): 111–123.
Properties of Landiolol hydrochloride
| Melting point: | 125.4° |
| storage temp. | Refrigerator |
| solubility | DMSO (Slightly), Ethanol (Slightly, Sonicated) |
| form | Solid |
| color | White to Off-White |
Safety information for Landiolol hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Landiolol hydrochloride
| InChIKey | DLPGJHSONYLBKP-WRNKUMIYNA-N |
| SMILES | C(C1C=CC(OC[C@@H](O)CNCCNC(N2CCOCC2)=O)=CC=1)CC(=O)OC[C@@H]1COC(C)(C)O1.Cl |&1:7,29,r| |
New Products
Bromine 99.5% AR (4 x 500ml) Fehling's Solution No. B Amino Acid Kit of 23 items set Ammonium Molybdate Reagent Solution Beam's Reagent Solution Ehrlich's Reagent For detection of urobillinogen 4-((2-isopropoxyethoxy)methyl)phenol 4-Hydroxy Carbazole Amino Salicylic Acid. U.S.P. 1,2,3,4-Tetrahydrocarbazol-4-one 2 – Methoxy – 5- Sulfamoyl Benzoic acid Acetone Isobutryl oxime ester Curcuma aromatica Oil Curry leaf Extract Terminalia bellirica Extract Aloe vera extract 200x Withania somnifera (Ashwagandha Extract) Citrus bioflavonoids Extract 5-Nitrosalicylaldehyde 5-(Difluoromethoxy)-2-Mercapto-1H-Benzimidazole- IP/BP/ Ethyl 3-(Pyridin-2-Ylamino)Propanoate Bilastine -IP/BP/ Cypermethric Acid Chloride Methyl Di Chloride (Mdc)Related products of tetrahydrofuran
You may like
-
 Landiolol Hydrochloride CAS 144481-98-1View Details
Landiolol Hydrochloride CAS 144481-98-1View Details
144481-98-1 -
 Landiolol HCl 98% (HPLC) CAS 144481-98-1View Details
Landiolol HCl 98% (HPLC) CAS 144481-98-1View Details
144481-98-1 -
 Landiolol hydrochloride CAS 144481-98-1View Details
Landiolol hydrochloride CAS 144481-98-1View Details
144481-98-1 -
 7726-95-6 Bromine 99.5% AR (4 x 500ml) 99%View Details
7726-95-6 Bromine 99.5% AR (4 x 500ml) 99%View Details
7726-95-6 -
 Formamide 99%View Details
Formamide 99%View Details
75-12-7 -
 85-81-4 6-Methoxy-8-Nitroquinoline 99%View Details
85-81-4 6-Methoxy-8-Nitroquinoline 99%View Details
85-81-4 -
![3-Bromo-4,5-Dihydro-1H-Benzo[B]Azepin-2(3H)-One 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 3-Bromo-4,5-Dihydro-1H-Benzo[B]Azepin-2(3H)-One 99%View Details
3-Bromo-4,5-Dihydro-1H-Benzo[B]Azepin-2(3H)-One 99%View Details
86499-96-9 -
 (−)-Dip-Chloride 85116-37-6 99%View Details
(−)-Dip-Chloride 85116-37-6 99%View Details
85116-37-6