L-xylulose
- CAS NO.:527-50-4
- Empirical Formula: C5H10O5
- Molecular Weight: 150.13
- MDL number: MFCD00064286
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-23 13:36:13
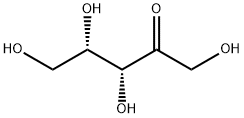
What is L-xylulose?
Chemical properties
L-xylulose is a clear liquid. It is a ketopentose, meaning that it has five carbon atoms and a ketogroup at carbon C-2. It has a molecular formula of C5H10O5 and a molar mass of 150.13 g/mol. Xylulose is almost colorless and it forms syrup. The formation of crystals has not been reported (Budavari, 1996). Both D- and L - enantiomers of xylulose are found as intermediates in metabolic pathways of prokaryotes as well as eukaryotes. Both forms are rare in nature (Doten and Mortlock, 1985b).
The Uses of L-xylulose
L-Xylulose is used in studies relating to potential inhibitors of glycosidases and has been proven to inhibit oligosaccharide processing to a degree.
What are the applications of Application
L-Xylulose is a monosaccharide
Definition
ChEBI: L-xylulose is a xylulose. It has a role as a human metabolite, an Escherichia coli metabolite and a mouse metabolite. It is an enantiomer of a D-xylulose.
What are the applications of Application
L-Xylulose has been shown to be a specific inhibitor of certain α-glucosidases,while having virtually no effect on other glycosidases such as β-glucosidase or α- and β-mannosidases. Furthermore,it is a specific inhibitor of the N-linked glycoproteinprocessing enzyme,glucosidase I, but does not inhibit glucosidase I or otherglycoprotein processing mannosidases. Thus it could prove to be a useful inhibitor forstudying glycoprotein processing, especially as it has been proven to be non-toxic andto also be effective in cell cultures (Muniruzzaman et al., 1996). L-Xylulose has been shown to have a strong inhibitory effect on the a-glucosidases,sucrase and maltase, present in the small intestine. Thus, an L-xylulose containing drug preparation for reducing blood sugar levels in humans and animals has been patented (Heinz et al.,1998).
Preparation
In 1933 a synthetic procedure for producing small amounts of D- and L -xylulose was introduced. In this method D- or L-xylose is epimerized to the corresponding diastereomer. The isomerization of xylose in pyridine leads to the production of xylulose (Touster, 1962). This enzyme catalyzes the production of L-xylulose from xylitol using either PQQ (pyrroloquinoline quinone) or FAD (flavin adenine dinucleotide) as prosthetic group, and L-xylulose is accumulated in the medium (Adachi et al., 1999; Adachi et al., 2001).
Properties of L-xylulose
| Melting point: | Not Applicable |
| Boiling point: | 469.1±45.0 °C(Predicted) |
| Density | 1.516±0.06 g/cm3(Predicted) |
| Flash point: | >230 °F |
| storage temp. | 2-8°C |
| solubility | Methanol (Slightly), Water (Soluble) |
| pka | 11.90±0.20(Predicted) |
| form | syrup |
| color | light yellow |
Safety information for L-xylulose
Computed Descriptors for L-xylulose
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1