L-(+)-Ergothioneine
Synonym(s):(S)-α-Carboxy-N,N,N-trimethyl-2-mercapto-1H-imidazole-4-ethanaminium inner salt;Thioneine
- CAS NO.:497-30-3
- Empirical Formula: C9H15N3O2S
- Molecular Weight: 229.3
- MDL number: MFCD00167474
- EINECS: 207-843-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
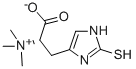
What is L-(+)-Ergothioneine?
Description
L-
Chemical properties
White Solid
The Uses of L-(+)-Ergothioneine
L-(+)-Ergothioneine is a natural molecule isolated from the rye ergot fungus and later identified in rat erythrocytes and liver and in numerous other animal tissues. Its antioxidizing properties may afford the compound therapeutic potential or it may be used as a food additive or in cosmetics.
What are the applications of Application
L-Ergothioneine is an antioxidant and free radical scavenger
Definition
ChEBI: Ergothioneine is a L-histidine derivative that is N(alpha),N(alpha),N(alpha)-trimethyl-L-histidine in which the hydrogen at position 2 on the imdazole ring is replaced by a mercapto group. A naturally occurring metabolite of histidine synthesized by bacteria and fungi with antioxidant properties. It is found ubiquitously in plants and animals and is present in many human foodstuffs. It has a role as an antioxidant, a fungal metabolite, a plant metabolite, a xenobiotic metabolite and a chelator. It is an amino-acid betaine, a L-histidine derivative and a sulfur-containing amino acid. It is a conjugate base of an ergothioneine(1+). It is a tautomer of an ergothioneine thione form.
Origin
Ergothioneine was discovered in 1909 by Charles Tanret, a French pharmacist and chemist. Tanret was examining the ergot fungus, which had recently been responsible for destroying crops, and he discovered the compound by using a purification process. The amino acid name ergothioneine originates from this fungus. Though this discovery is relatively recent, scientists speculate that ergothioneine may have originated from ancient earth. Due to its anaerobic nature (it does not require oxygen to function), it may have manifested in the earth's oxygen-free atmosphere more than three billion years ago While ergothioneine is not classified as one of the nine essential amino acids.
Benefits
L-(+)-Ergothioneine is a natural antioxidant, which has various physiological functions such as scavenging free radicals, detoxification, maintaining DNA biosynthesis, normal cell growth and cellular immunity.
General Description
L-(+)-Ergothioneine (ET) is a sulfur-containing amino acid, which is only produced by Actinomycetales bacteria and non-yeast like fungi belonging to the division Basidiomycota and Ascomycota. It was originally isolated from Claviceps purpurea or rye ergot. It is obtained from L-histidine, which is converted into betaine form called hercynine. It is found in both animals and plants, and mammals usually obtain it from their diet, e.g. through mushrooms or oats. It is tautomeric in nature, and in neutral aqueous solution exists in thione form.
General Description
L-(+)-Ergothioneine (ERG) is a substance that cannot be synthesized by humans and must be obtained from food. It has cytoprotective and antioxidant properties.
Biochem/physiol Actions
L-(+)-Ergothioneine (ET) has the maximum concentrations in tissues subjected to oxidative stress, with the highest being in blood, eye lens, bone marrow, semen and liver. It acts as an anti-oxidant and prevents apoptosis, by scavenging reactive oxygen and nitrogen species. The anti-oxidant activity is attributable to sulfhydryl groups. It acts as a substrate for SLC22A4 (solute carrier family 22, member 4) transporter. In alveolar macrophages, it prevents the release of interleukin-8 (IL-8) by tumor necrosis factor (TNF)α. IL-8 is an inflammatory cytokine. It also regulates the oxidative damage in liver and kidneys, and has a protective action against lipid peroxidation. It is also responsible for the conservation of endogenous glutathione and α-tocopherol. ET being an antioxidant, protects against γ and UV radiation. In UV-irradiated human dermal fibroblasts, it scavenges reactive oxygen species (ROS), and suppresses matrix metalloproteinases 1 (MMP1) expression. It might also have anti-ageing effects on skin caused by UV-radiation.
Synthesis
The synthesis of L-(+)-Ergothioneine (ET; marketed as Ergoneine®) is performed in water and includes the following steps. First, l-hercynine is reacted with bromine and then with cysteine. The intermediate obtained is transformed into ET by heating in the presence of mercaptopropionic acid. Finally, the raw product is purified by crystallisation.
Synthesis
L-(+)-Ergothioneine is prepared by the reaction of hercynine. The steps are as follows:
15g of compound was added to 150ml of water, and 15.6g of concentrated hydrochloric acid was added, add 10.9g dibromohydantoin, stir for 20min, add D-cysteine, Continue to stir for 1 hour, add sodium thiosulfate, raise the temperature to 90-100°C, and continue the reaction for 15 hours. After the reaction, cool down and filter, adjust the pH to neutral, desalt, concentrate, crystallize with 5ml of water and 75ml of isopropanol, and dissolve the solid Filter and dry at 70-80°C to obtain 88% ergothioneine product with a yield of 81%.
References
[1] Williamson R, et al. L-(+)-Ergothioneine Significantly Improves the Clinical Characteristics of Preeclampsia in the Reduced Uterine Perfusion Pressure Rat Model. Hypertension , 2022; 75.
[2] Hoek S, et al. Engineering the Yeast Saccharomyces cerevisiae for the Production of L-(+)-Ergothioneine. Frontiers in Bioengineering and Biotechnology, 2019.
Properties of L-(+)-Ergothioneine
| Melting point: | 275-277°C (dec.) |
| Density | 1.2541 (rough estimate) |
| refractive index | 1.6740 (estimate) |
| storage temp. | -20°C |
| solubility | Soluble in Water (up to 10 mg/ml) |
| form | White solid. |
| color | White or off-white |
| PH | +47^o (c=1 in water) |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in water may be stored at -20°C for no more then 1 day. |
| InChI | InChI=1/C9H15N3O2S/c1-12(2,3)7(8(13)14)4-6-5-10-9(15)11-6/h5,7H,4H2,1-3H3,(H2-,10,11,13,14,15)/t7-/s3 |
| EPA Substance Registry System | 1H-Imidazole-4-ethanaminium, .alpha.-carboxy-2,3-dihydro-N,N,N-trimethyl-2-thioxo-, inner salt, (.alpha.S)- (497-30-3) |
Safety information for L-(+)-Ergothioneine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. |
Computed Descriptors for L-(+)-Ergothioneine
| InChIKey | SSISHJJTAXXQAX-KPOCXSGKNA-N |
| SMILES | [N+](C)(C)(C)[C@@H](CC1=CNC(S)=N1)C([O-])=O |&1:4,r| |
L-(+)-Ergothioneine manufacturer
Anthem Biosciences Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 497-30-3 L-(+)-Ergothioneine 99%View Details
497-30-3 L-(+)-Ergothioneine 99%View Details
497-30-3 -
 L-(+)-Ergothioneine CAS 497-30-3View Details
L-(+)-Ergothioneine CAS 497-30-3View Details
497-30-3 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1