L-CYSTATHIONINE
Synonym(s):(R)-S-(2-Amino-2-carboxyethyl)-L -homocysteine
- CAS NO.:56-88-2
- Empirical Formula: C7H14N2O4S
- Molecular Weight: 222.26
- MDL number: MFCD00036685
- EINECS: 200-295-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
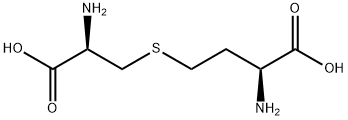
What is L-CYSTATHIONINE?
Description
L-
Chemical properties
White Crystalline Solid
The Uses of L-CYSTATHIONINE
L-Cystathionine has been used in ultra-performance liquid chromatography for the validation of cystathionine and the correlation of its abundance with vertebrate metamorphosis. It has also been used as an inhibitor of sinusoidal GSH (glutathione) carrier to signify that thiol redox state imbalance is associated with the modulation of autophagy in carcinoma cells.
The Uses of L-CYSTATHIONINE
Intermediate in transsulfuration whereby the mammal transfers the sulfur of methionine via homocysteine to cysteine
What are the applications of Application
L-(+)-Cystathionine is an intermediate in transsulfuration
Definition
ChEBI: L-cystathionine is a modified amino acid generated by enzymic means from L-homocysteine and L-serine. It has a role as a human metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite and a mouse metabolite. It is a tautomer of a L-cystathionine dizwitterion.
Biochem/physiol Actions
L-Cystathionine is an intermediate in the biosynthesis of L-cysteine and methionine. It is used as a substrate to differentiate and analyze cystathionine γ-lyase(s). L-Cystathionine is an important non-protein amino acid and is associated with metabolic disorders. In recent studies, L-cystathionine is believed to be the precursor of cyclic imino acid cyclothionine. Cystathioninuria is characterized with imino acid cyclothionine excretion in the urine.
Purification Methods
S,S-Cystathionine is purified by converting it to the HCl salt in 20% HCl and carefully basifying with aqueous NH3 until separation is complete. Filter it off and dry it in a vacuum. It forms prisms from H2O. The dibenzoyl derivative has m 229o (from EtOH). [IR: Greenstein & Winitz Chemistry of the Amino Acids (J Wiley) Vol 3 p2690 1961 and Tallan et al. J Biol Chem 230 707 1958, Synthesis: du Vigneaud et al. J Biol Chem 143 59 1942, Anslow et al. J Biol Chem 166 39 1946.] [Prepn: Weiss & Stekol J Am Chem Soc 73 2497 1951; see also du Vigneaud et al. J Biol Chem 143 60 1942, Biological synthesis: Greenberg Methods Enzymol 5 943 1962, Beilstein 4 IV 3197.]
Properties of L-CYSTATHIONINE
| Melting point: | 312 |
| alpha | D20 +23.7° (1N HCl) |
| Density | 1.430±0.06 g/cm3 (20 ºC 760 Torr) |
| storage temp. | -20°C |
| solubility | Aqueous Acid (Slightly) |
| form | Solid |
| Boiling point: | 481.0±45.0 °C(Predicted) |
| pka | 2.08±0.10(Predicted) |
| color | White to Off-White |
| Merck | 13,2807 |
| BRN | 2505200 |
Safety information for L-CYSTATHIONINE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for L-CYSTATHIONINE
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



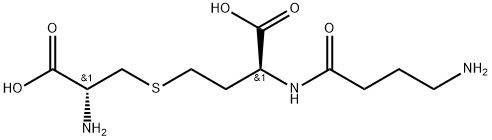
![BUTANOIC ACID, 2-AMINO-4-[(S)-[(2R)-2-AMINO-2-CARBOXYETHYL]SULFINYL]-, (2S)-](https://img.chemicalbook.in/CAS/GIF/210647-25-9.gif)


You may like
-
 L-Cystathionine CAS 56-88-2View Details
L-Cystathionine CAS 56-88-2View Details
56-88-2 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1