L-165,041
Synonym(s):4-[3-(4-Acetyl-3-hydroxy-2-propylphenoxy)propoxy]phenoxy-acetic acid;Compound P, L165041, 4-[3-(2-Propyl-3-hydroxy-4-acetyl)phenoxy]propyloxyphenoxy-acetic acid, PPAR Agonist VIII, PPARα Agonist III, PPARγ Agonist VII;L-165,041 - CAS 79558-09-1 - Calbiochem
- CAS NO.:79558-09-1
- Empirical Formula: C22H26O7
- Molecular Weight: 402.44
- MDL number: MFCD04974501
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
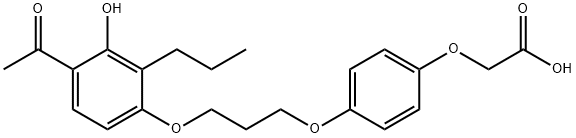
What is L-165,041?
The Uses of L-165,041
L-165,041 has been used as a peroxisome proliferator-activated receptor β/δ (PPARβ/δ) ligand to study its influence on PPARβ/δ mediated postnatal myogenesis in C2C12 myoblasts.
What are the applications of Application
L-165041 is a cell permeable peroxisome proliferator activator receptor b agonist
Definition
ChEBI: 2-[4-[3-(4-acetyl-3-hydroxy-2-propylphenoxy)propoxy]phenoxy]acetic acid is an aromatic ketone.
General Description
A cell-permeable phenoxyacetic acid derivative that acts as a potent and selective peroxisome proliferator activator receptor δ (PPARδ) agonist (Ki = 6 nM for hPPARδ and 730 nM for hPPARγ). Potently induces adipocyte differentiation in NIH-PPARδ cells at 500 nM and raises total cholesterol in insulin resistant db/db mice without altering glucose or triglycerides levels. Increases UCP3 (uncoupling protein 3) gene expression in L6 myotubes. Inhibits cytokine-induced expression of VCAM-1 (vascular cell adhesion molecule-1) and the secretion of MCP-1 (monocyte chemotactic protein-1) in EAhy926 cells.
Biological Activity
Potent PPAR δ agonist (K i = 6 nM); displays > 100-fold selectivity for both mouse and human PPAR δ ? receptors over other subtypes. In vivo, raises plasma cholesterol levels in insulin-resistant db/db mice and is neuroprotective in models of cerebral infarction and Parkinson's disease.
Biochem/physiol Actions
Cell permeable: yes
in vitro
l-165041, which is a selective and potent pparδligand, displayed in this specified transactivation system, apart from its highly efficacious pparδ agonist activity, partial and full agonism at, respectively, pparγ2 and pparαsubtypes [1].
in vivo
l-165041 could drastically reduce lipid accumulation in the mouse liver, decreasing total hepatic triglyceride and cholesterol content compared to the vehicle group. gene analysis demonstrated that l-165041 lowered hepatic expression of pparγ, apolipoprotein b, il-1β, and interleukin-6. in contrast, l-165041 increased hepatic expressions of pparδ, lipoprotein lipase, and atp-binding cassette transporter g1 (abcg1) [2].
storage
Desiccate at RT
References
[1] wurch t, junquero d, delhon a, pauwels j. pharmacological analysis of wild-type alpha, gamma and delta subtypes of the human peroxisome proliferator-activated receptor. naunyn schmiedebergs arch pharmacol. 2002 feb;365(2):133-40.
[2] lim hj, park jh, lee s, choi he, lee ks, park hy. ppardelta ligand l-165041 ameliorates western diet-induced hepatic lipid accumulation and inflammation in ldlr-/- mice. eur j pharmacol. 2009 nov 10;622(1-3):45-51.
Properties of L-165,041
| Melting point: | 127-128 °C(lit.) |
| Boiling point: | 600.8±55.0 °C(Predicted) |
| Density | 1.222±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: >10 mg/mL |
| form | solid |
| pka | 3.23±0.10(Predicted) |
| color | off-white |
| Stability: | Light Sensitive |
Safety information for L-165,041
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for L-165,041
New Products
4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION Methylcobalamin (vitamin B12) SODIUM METHYL PARABEN SODIUM VALPROATE AMOXICILLIN (AMOXYCILLIN) TRIHYDRATE ACICLOVIRRelated products of tetrahydrofuran
You may like
-
 L-165,041 CAS 79558-09-1View Details
L-165,041 CAS 79558-09-1View Details
79558-09-1 -
 L-165,041 CAS 79558-09-1View Details
L-165,041 CAS 79558-09-1View Details
79558-09-1 -
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9