KRYPTON
- CAS NO.:7439-90-9
- Empirical Formula: Kr
- Molecular Weight: 83.8
- MDL number: MFCD00016158
- EINECS: 231-098-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-04 15:07:40
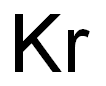
What is KRYPTON?
Description
Krypton, Kr, is an elemental, colorless, odorless inert gas. It is noncombustible, nontoxic, and nonreactive; however, it is an asphyxiant gas and will displace oxygen in the air. Krypton 85 is radioactive and has a half-life of 10.3 years. The four-digit UN identification number for krypton is 1056 as a compressed gas and 1970 as a cryogenic liquid. These forms of krypton are not radioactive. Radioactive isotopes of krypton are shipped under radioactive labels and placards as required. Its primary uses are in the activation of phosphors for self-luminous markers, detecting leaks, and in medicine to trace blood flow.
Description
I’m a real element that has a fictional counterpart. What atom am I?
Krypton is an inert (or “noble”) gas and, as such, is not bound up in molecules but exists as single atoms.1 It is the fourth atom in group 18 of the periodic table of elements, after helium, neon, and argon. Its concentration in Earth’s atmosphere is ≈1 ppm by volume.
In 1898, British chemists William Ramsay and Morris Travers discovered krypton as the residue of evaporating almost all of the other components of liquid air. For his work in the discovery of several inert gases, Ramsay was awarded the Nobel Prize in Chemistry in 1904.
Like all inert gases, the boiling and melting points of krypton are only a few degrees apart (see “fast facts”). Krypton has few practical applications, mostly in bright white light bulbs used in photography and in devices used in physical and chemical research. At one time, the wavelength of light emitted by the 86Kr isotope was used to define the meter, but in 1983, the meter’s definition was changed to a function of the speed of light.
In comic books dating back to 1949, “Krypton” was the planet where Superman was born; and “kryptonite” was the only substance that the superhero was vulnerable to. Over the years, the mythological kryptonite was endowed with much more extensive chemistry than real krypton gas.
1. Although there are exceptions, such as former Molecule of the Week krypton difluoride.
Chemical properties
colourless gas
Physical properties
Krypton is a rather dense, tasteless, colorless, odorless gas. Its critical temperature isbetween that of oxygen and carbon dioxide. It is extracted during fractional distillation ofliquid oxygen at a temperature of about –63.8°C. At one time it was thought that krypton, aswell as the other noble gases, were completely inert. However, in 1967 scientists were able tocombine fluorine with krypton at low temperatures to form the compound krypton difluoride(KrF2). In this case krypton has a valence of 2.
Krypton’s melting point is –156.6°C, its boiling point is –152.30°C, and its density is0.003733g/cm3.
Isotopes
There are a total of 37 isotopes of krypton. Six of these are stable: Kr-78, Kr-80,Kr-82, Kr-83, Kr-84, and Kr-86. The isotope Kr-78 has such a long half-life (0.9×10+20years) that it is considered stable even though it contributes only 0.35% to the naturalkrypton in the Earth’s atmosphere. All the others are radioactive, man-made by-productsof nuclear power plants and radioactive isotopes with half-lives ranging from 107 nanosecondsto 2.29×10+15 years.
Origin of Name
The name “krypton” is derived from the Greek word kryptos, meaning “hidden.”
Occurrence
Krypton is the 81st most abundant element on Earth and ranks seventh in abundance ofthe gases that make up Earth’s atmosphere. It ranks just above methane (CH4) in abundancein the atmosphere. Krypton is expensive to produce and thus has limited use. The gas is capturedcommercially by fractional distillation of liquid air. Krypton shows up as an impurity inthe residue. Along with some other gases, it is removed by filtering through activated charcoaland titanium.
There are traces of krypton in some minerals and meteorites. Krypton is found beyondEarth in space.
History
Discovered in 1898 by Ramsay and Travers in the residue left after liquid air had nearly boiled away, krypton is present in the air to the extent of about 1 ppm. The atmosphere of Mars has been found to contain 0.3 ppm of krypton. It is one of the “noble” gases. It is characterized by its brilliant green and orange spectral lines. Naturally occurring krypton contains six stable isotopes. Thirty other unstable isotopes and isomers are now recognized. The spectral lines of krypton are easily produced and some are very sharp. In 1960 it was internationally agreed that the fundamental unit of length, the meter, should be defined in terms of the orange-red spectral line of 86Kr. This replaced the standard meter of Paris, which was defined in terms of a bar made of a platinum-iridium alloy. In October 1983 the meter was again redefined by the International Bureau of Weights and Measures as being the length of path traveled by light in a vacuum during a time interval of 1/299,792,458 of a second. Solid krypton is a white crystalline substance with a face-centered cubic structure that is common to all the rare gases. While krypton is generally thought of as a noble gas that normally does not combine with other elements, the existence of some krypton compounds has been established. Krypton difluoride has been prepared in gram quantities and can be made by several methods. A higher fluoride of krypton and a salt of an oxyacid of krypton also have been prepared. Molecule-ions of ArKr+ and KrH+ have been identified and investigated, and evidence is provided for the formation of KrXe or KrXe+. Krypton clathrates have been prepared with hydroquinone and phenol. 85Kr has found recent application in chemical analysis. By imbedding the isotope in various solids, kryptonates are formed. The activity of these kryptonates is sensitive to chemical reactions at the surface. Estimates of the concentration of reactants are therefore made possible. Krypton is used in certain photographic flash lamps for highspeed photography. Uses thus far have been limited because of its high cost. Krypton gas presently costs about $690/100 L.
Characteristics
Krypton is the fourth element in group 18 (VIIIA), which is also known as group 0 becausethe elements is this group were thought to have a zero oxidation point. Krypton has many ofthe chemical properties and characteristics of some of the other noble gases.
The fragile compounds formed by noble gases at low temperatures, such as KrF2, are calledclathrates.
The Uses of KRYPTON
Krypton is expensive to produce, which limits its use as an inert gas. It is used in a mixturewith argon to fill incandescent light bulbs, fluorescent lamps, lasers, and high-speed photographylamps. Radioactive Kr-85 is used as a source of radiation to measure the thickness ofindustrial materials. It is also used to test for “leakage” of scientific instruments.
Since 1960 the wavelength of the spectral lines of the krypton-86 isotope has been usedas the standard for the length of the meter. One meter is now defined as 1,650,762.73 wavelengthsof the reddish-orange spectral line of the Kr-86 isotope.
The Uses of KRYPTON
Krypton is a colourless and odourless rare gas that is heavier than air. Applications of krypton include laser technology, lighting, and use as an insulating gas (e.g. for windows). Gas is easy to handle in a small, light pressure can, while the disposable container allows for aluminium recycling when finished.
Definition
A colorless odorless monatomic element of the rare-gas group, known to form unstable compounds with fluorine. It occurs in minute quantities (0.001% by volume) in air. Krypton is used in fluorescent lights. Symbol: Kr; m.p. –156.55°C; b.p. –152.3°C; d. 3.749 (0°C) kg m–3; p.n. 36; r.a.m. 83.80.
Definition
krypton: Symbol Kr. A colourlessgaseous element belonging to group0 (the noble gases) of the periodictable; a.n. 36; r.a.m. 83.80; d. 3.73gm-3; m.p.-156.6°C; b.p.-152.3°C.Krypton occurs in air (0.0001% by volume)from which it can be extractedby fractional distillation of liquid air.Usually, the element is not isolatedbut is used with other inert gases influorescent lamps, etc. The element has five natural isotopes (mass num-bers 78, 80, 82, 83, 84) and there are five radioactive isotopes (76, 77, 79,81, 85). Krypton-85 (half-life 10.76years) is produced in fission reactorsand it has been suggested that anequilibrium amount will eventuallyoccur in the atmosphere. The elementis practically inert and formsvery few compounds (certain fluorides, such as KrF2, have been reported).
Production Methods
Most krypton produced in commercial scale comes from air. Krypton and other inert gases are obtained from air by a distillation-liquefaction process. Different types of air-separation plants varying in design are known for commercial production of nitrogen, oxygen, and inert gases (See Helium).
Krypton also may be recovered from spent fuel rods of nuclear power plants. It is produced, along with xenon, in fission of uranium and plutonium. This process, however, is not a major source of krypton, and the recovered gas also contains radioactive Kr-85 isotope.
.
General Description
Krypton, refrigerated liquid, is a colorless, odorless gas. KRYPTON is shipped as a liquid under its own vapor pressure. Contact with the liquid may cause frostbite to unprotected skin. KRYPTON can asphyxiate by displacement of air. Exposure of the container to prolonged heat or fire may cause KRYPTON to rupture violently and rocket.
Reactivity Profile
These substances undergo no chemical reactions under any known circumstances. They are nonflammable, noncombustible and nontoxic. They can asphyxiate. Contact of very cold liquefied gas with water may result in vigorous or violent boiling of the product and extremely rapid vaporization due to the large temperature differences involved. If the water is hot, there is the possibility that a liquid "superheat" explosion may occur. Pressures may build to dangerous levels if liquid gas contacts water in a closed container [Handling Chemicals Safely 1980].
Hazard
Being an inert gas, krypton is nontoxic. However, the man-made radioisotopes of kryptoncan cause radiation poisoning.
Health Hazard
Vapors may cause dizziness or asphyxiation without warning. Vapors from liquefied gas are initially heavier than air and spread along ground. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite.
Fire Hazard
Non-flammable gases. Containers may explode when heated. Ruptured cylinders may rocket.
Industrial uses
Krypton, which occurs in the air to the extentof 1 part in 1 million, is a heavy gas used as afiller for fluorescent lamps to decrease filamentevaporation and heat loss and to permit highertemperatures in the lamp. Krypton-85, obtainedfrom atomic reactions, is a beta-ray emitter usedin luminous paints for activating phosphors andalso as a source of radiation.
Properties of KRYPTON
| Melting point: | -157℃ |
| Boiling point: | bp -153.35° |
| Density | 908 kg/m3; d0 (101.3 kPa) 3.7493 kg/m3; d (normal bp) 8.6 kg/m3; d (normal bp) 2415 kg/m3; d (triple pt) 2451 kg/m3; d (triple pt) 2826 kg/m3 |
| solubility | ≈7 x 10–5 ml/l or
≈3 x 10–7 mg/l |
| solubility | slightly soluble in H2O |
| appearance | colorless gas |
| form | colorless gas |
| color | colorless |
| Water Solubility | 59.4mL/1000g H2O (20°C) [KIR78]; Henry’s law constants, k×10?4: 3.685 (70.2°C), 4.017 (175.0°C), 3.761 (175.0°C), 2.392 (252.5°C) [POT78] |
| Merck | 13,5340 |
| Stability: | Stable and unreactive; not combustible. |
| EPA Substance Registry System | Krypton (7439-90-9) |
Safety information for KRYPTON
| Signal word | Warning |
| Pictogram(s) |
 Gas Cylinder Compressed Gases GHS04 |
| GHS Hazard Statements |
H280:Gases under pressure |
| Precautionary Statement Codes |
P410+P403:Protect from sunlight. Store in a well-ventilated place. |
Computed Descriptors for KRYPTON
New Products
BOC-L-4-HYDROXYPROLINE 2-nitro 3-hydroxy pyridine 2,6-Dichloropyridin-4-amine 2,3 Diamino pyridine 5-Iodo-2-(1-methylethyl)-3(2H)-pyridazinone 1-Azetidinecarboxylic acid, 3-[(3S)-1-(trans-3-carboxy-3-methylcyclobutyl)-3-piperidinyl]-, 1-(1,1-dimethylethyl) ester Tert-Butyl N-[3-(dimethylcarbamoyl)prop-2-en-1-yl]carbamate 1-(difluoromethyl)-N-methylcyclobutan-1-amine 2-(4-Methyl-1,2,5-oxadiazol-3-yl)-1H-benzimidazole 6-(4-iodophenyl)-1-oxa-6-azaspiro[3.3]he Trimethyl(phenylthio)silane Polycaprolactone(2000)-PEG(20000)-Polycaprolactone(2000) Diacrylate Diethylene Glycol Monoethyl Ether, PolyoxyethyleneOleylCetylEtherSulfosuccinate Ascorbyl Tetraisopalmitate or Tetrahexyldecyl Ascorbate Castor Oil, Ethoxylated, Cremophor EL or PEG-35 Castor Oil Tween 20 or Polysorbate 20 Acetone-d6 (R)-2-Mercaptobutanoic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine 3-(naphthalen-1-ylsulfonyl)-1H-indazol-5-amine methyl 5-amino-3-(1,1-dioxidotetrahydro-2H-1,2-thiazin-2-yl)-2-fluorobenzoate 7-methoxy-8-(2-morpholinoethoxy)-4-((3,4,5-trimethoxyphenyl)amino)benzo[g]quinoline-3-carbonitrile Dimethylaluminum isopropoxideRelated products of tetrahydrofuran
You may like
-
 7439-90-9 Krypton 98%View Details
7439-90-9 Krypton 98%View Details
7439-90-9 -
 7439-90-9 98%View Details
7439-90-9 98%View Details
7439-90-9 -
 Krypton CAS 7439-90-9View Details
Krypton CAS 7439-90-9View Details
7439-90-9 -
 121-54-0 Benzethonium Chloride 98%View Details
121-54-0 Benzethonium Chloride 98%View Details
121-54-0 -
 5721-91-5 98%View Details
5721-91-5 98%View Details
5721-91-5 -
 Calcium 11% clear solution 98%View Details
Calcium 11% clear solution 98%View Details -
 Sodium Croscarmellose 98%View Details
Sodium Croscarmellose 98%View Details
74811-65-7 -
 16455-61-1 98%View Details
16455-61-1 98%View Details
16455-61-1







